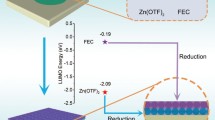
Abstract
An interlayer is usually employed to tackle the interfacial instability issue between solid electrolytes (SEs) and Li metal caused by the side reaction. However, the failure mechanism of the ionic conductor interlayers, especially the influence from electron penetration, remains largely unknown. Herein, using Li1.3Al0.3Ti1.7(PO4)3 (LATP) as the model SE and LiF as the interlayer, we use metal semiconductor contact barrier theory to reveal the failure origin of Li/LiF@LATP interface based on the calculation results of density functional theory (DFT), in which electrons can easily tunnel through the LiF grain boundary with F vacancies due to its narrow barrier width against electron injection, followed by the reduction of LATP. Remarkably, an Al-LiF bilayer between Li/LATP is found to dramatically promote the interfacial stability, due to the highly increased barrier width and homogenized electric field at the interface. Consequently, the Li symmetric cells with Al-LiF bilayer can exhibit excellent cyclability of more than 2,000 h superior to that interlayered by LiF monolayer (∼ 860 h). Moreover, the Li/Al-LiF@LATP/LiFePO4 solid-state batteries deliver a capacity retention of 83.2% after 350 cycles at 0.5 C. Our findings emphasize the importance of tuning the electron transport behavior by optimizing the potential barrier for the interface design in high-performance solid-state batteries.

Similar content being viewed by others
References
Lee, M. J.; Han, J.; Lee, K.; Lee, Y. J.; Kim, B. G.; Jung, K. N.; Kim, B. J.; Lee, S. W. Elastomeric electrolytes for high-energy solid-state lithium batteries. Nature 2022, 601, 217–222.
Wang, C. H.; Yu, R. Z.; Duan, H.; Lu, Q. W.; Li, Q. Z.; Adair, K. R.; Bao, D. N.; Liu, Y.; Yang, R.; Wang, J. T. et al. Solvent-free approach for interweaving freestanding and ultrathin inorganic solid electrolyte membranes. ACS Energy Lett. 2022, 7, 410–416.
Chi, X. W.; Li, M. L.; Di, J. C.; Bai, P.; Song, L. N.; Wang, X. X.; Li, F.; Liang, S.; Xu, J. J.; Yu, J. H. A highly stable and flexible zeolite electrolyte solid-state Li-air battery. Nature 2021, 592, 551–557.
Pang, Y. P.; Pan, J. Y.; Yang, J. H.; Zheng, S. Y.; Wang, C. S. Electrolyte/electrode interfaces in all-solid-state lithium batteries: A review. Electrochem. Energy Rev. 2021, 4, 169–193.
Weiss, M.; Simon, F. J.; Busche, M. R.; Nakamura, T.; Schröder, D.; Richter, F. H.; Janek, J. From liquid- to solid-state batteries: Ion transfer kinetics of heteroionic interfaces. Electrochem. Energy Rev. 2020, 3, 221–238.
Payandeh, S.; Strauss, F.; Mazilkin, A.; Kondrakov, A.; Brezesinski, T. Tailoring the LiNbO3 coating of Ni-rich cathode materials for stable and high-performance all-solid-state batteries. Nano Res. Energy 2022, 1, e9120016.
Guo, X.; Wang, C. D.; Wang, W. J.; Zhou, Q.; Xu, W. J.; Zhang, P. J.; Wei, S. Q.; Cao, Y. Y.; Zhu, K. F.; Liu, Z. F. et al. Vacancy manipulating of molybdenum carbide MXenes to enhance faraday reaction for high performance lithium-ion batteries. Nano Res. Energy 2022, 1, e9120026.
Li, C.; Liu, B. W.; Jiang, N. Y.; Ding, Y. Elucidating the chargetransfer and Li-ion-migration mechanisms in commercial lithium-ion batteries with advanced electron microscopy. Nano Res. Energy 2022, 1, e9120031.
Liu, Q.; Hu, Y. H.; Yu, X. R.; Qin, Y. F.; Meng, T.; Hu, X. L. The pursuit of commercial silicon-based microparticle anodes for advanced lithium-ion batteries: A review. Nano Res. Energy 2022, 1, e9120037.
Wang, Y.; Zanelotti, C. J.; Wang, X. E.; Kerr, R.; Jin, L. Y.; Kan, W. H.; Dingemans, T. J.; Forsyth, M.; Madsen, L. A. Solid-state rigidrod polymer composite electrolytes with nanocrystalline lithium ion pathways. Nat. Mater. 2021, 20, 1255–1263.
Christie, A. M.; Lilley, S. J.; Staunton, E.; Andreev, Y. G.; Bruce, P. G. Increasing the conductivity of crystalline polymer electrolytes. Nature 2005, 433, 50–53.
Tan, D. H. S.; Chen, Y. T.; Yang, H. D.; Bao, W.; Sreenarayanan, B.; Doux, J. M.; Li, W. K.; Lu, B. Y.; Ham, S. Y.; Sayahpour, B. et al. Carbon-free high-loading silicon anodes enabled by sulfide solid electrolytes. Science 2021, 373, 1494–1499.
Lu, P. S.; Liu, L. L.; Wang, S.; Xu, J. R.; Peng, J.; Yan, W. L.; Wang, Q. C.; Li, H.; Chen, L. Q.; Wu, F. Superior all-solid-state batteries enabled by a gas-phase-synthesized sulfide electrolyte with ultrahigh moisture stability and ionic conductivity. Adv. Mater. 2021, 33, 2100921.
Xu, L. Q.; Li, J. Y.; Deng, W. T.; Shuai, H. L.; Li, S.; Xu, Z. F.; Li, J. H.; Hou, H. S.; Peng, H. J.; Zou, G. Q. et al. Garnet solid electrolyte for advanced all-solid-state Li batteries. Adv. Energy Mater. 2021, 11, 2000648.
Samson, A. J.; Hofstetter, K.; Bag, S.; Thangadurai, V. A bird’s-eye view of Li-stuffed garnet-type Li7La3Zr2O12 ceramic electrolytes for advanced all-solid-state Li batteries. Energy Environ. Sci. 2019, 12, 2957–2975.
He, L. C.; Oh, J. A. S.; Watarai, K.; Morita, M.; Zhao, Y.; Sun, Q. M.; Sakamoto, T.; Lu, L.; Adams, S. Electromechanical failure of NASICON-type solid-state electrolyte-based all-solid-state Li-ion batteries. Chem. Mater. 2021, 33, 6841–6852.
Zhao, R.; Gao, L.; Song, M. R.; Ye, Y.; Liang, Z. B.; Bian, J. C.; Zhu, J. L.; Li, S.; Zou, R. Q.; Zhao, Y. S. Stabilization of NASICON-type electrolyte against Li anode via an ionic conductive MOF-incorporated adhesive interlayer. ACS Energy Lett. 2021, 6, 3141–3150.
Manthiram, A.; Yu, X. W.; Wang, S. F. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 16103.
Gao, Z. H.; Sun, H. B.; Fu, L.; Ye, F. L.; Zhang, Y.; Luo, W.; Huang, Y. H. Promises, challenges, and recent progress of inorganic solid-state electrolytes for all-solid-state lithium batteries. Adv. Mater. 2018, 30, 1705702.
Ye, L. H.; Li, X. A dynamic stability design strategy for lithium metal solid state batteries. Nature 2021, 593, 218–222.
Lee, Y. G.; Fujiki, S.; Jung, C.; Suzuki, N.; Yashiro, N.; Omoda, R.; Ko, D. S.; Shiratsuchi, T.; Sugimoto, T.; Ryu, S. et al. High-energy long-cycling all-solid-state lithium metal batteries enabled by silver-carbon composite anodes. Nat. Energy 2020, 5, 299–308.
Zhu, J. P.; Zhao, J.; Xiang, Y. X.; Lin, M.; Wang, H. C.; Zheng, B. Z.; He, H. J.; Wu, Q. H.; Huang, J. Y.; Yang, Y. Chemomechanical failure mechanism study in NASICON-type Li1.3Al0.3Ti1.7(PO4)3 solid-state lithium batteries. Chem. Mater. 2020, 32, 4998–5008.
Lewis, J. A.; Cortes, F. J. Q.; Boebinger, M. G.; Tippens, J.; Marchese, T. S.; Kondekar, N.; Liu, X. M.; Chi, M. F.; McDowell, M. T. Interphase morphology between a solid-state electrolyte and lithium controls cell failure. ACS Energy Lett. 2019, 4, 591–599.
Chung, H. B.; Kang, B. Mechanical and thermal failure induced by contact between a Li1.5Al0.5Ge1.5(PO4)3 solid electrolyte and Li metal in an all solid-state Li cell. Chem. Mater. 2017, 29, 8611–8619.
Zhang, Z. H.; Zhao, Y. R.; Chen, S. J.; Xie, D. J.; Yao, X. Y.; Cui, P.; Xu, X. X. An advanced construction strategy of all-solid-state lithium batteries with excellent interfacial compatibility and ultralong cycle life. J. Mater. Chem. A 2017, 5, 16984–16993.
Tippens, J.; Miers, J. C.; Afshar, A.; Lewis, J. A.; Cortes, F. J. Q.; Qiao, H. P.; Marchese, T. S.; Di Leo, C. V.; Saldana, C.; McDowell, M. T. Visualizing chemomechanical degradation of a solid-state battery electrolyte. ACS Energy Lett. 2019, 4, 1475–1483.
Liu, Y. J.; Li, C.; Li, B. J.; Song, H. C.; Cheng, Z.; Chen, M. R.; He, P.; Zhou, H. S. Germanium thin film protected lithium aluminum germanium phosphate for solid-state Li batteries. Adv. Energy Mater. 2018, 8, 1702374.
Cortes, F. J. Q.; Lewis, J. A.; Tippens, J.; Marchese, T. S.; McDowell, M. T. How metallic protection layers extend the lifetime of NASICON-based solid-state lithium batteries. J. Electrochem. Soc. 2020, 167, 050502.
Zhang, Z. H.; Chen, S. J.; Yang, J.; Liu, G. Z.; Yao, X. Y.; Cui, P.; Xu, X. X. Stable cycling of all-solid-state lithium battery with surface amorphized Li1.5Al0.5Ge1.5(PO4)3 electrolyte and lithium anode. Electrochim. Acta 2019, 297, 281–287.
Liu, Y. L.; Sun, Q.; Zhao, Y.; Wang, B. Q.; Kaghazchi, P.; Adair, K. R.; Li, R. Y.; Zhang, C.; Liu, J. R.; Kuo, L. Y. et al. Stabilizing the interface of NASICON solid electrolyte against Li metal with atomic layer deposition. ACS Appl. Mater. Interfaces 2018, 10, 31240–31248.
Cheng, Q.; Li, A. J.; Li, N.; Li, S.; Zangiabadi, A.; Li, T. D.; Huang, W. L.; Li, A. C.; Jin, T. W.; Song, Q. Q. et al. Stabilizing solid electrolyte-anode interface in Li-metal batteries by boron nitride-based nanocomposite coating. Joule 2019, 3, 1510–1522.
Hao, X. G.; Zhao, Q.; Su, S. M.; Zhang, S. Q.; Ma, J. B.; Shen, L.; Yu, Q. P.; Zhao, L.; Liu, Y.; Kang, F. Y. et al. Constructing multifunctional interphase between Li1.4Al0.4Ti1.6(PO4)3 and Li metal by magnetron sputtering for highly stable solid-state lithium metal batteries. Adv. Energy Mater. 2019, 9, 1901604.
Huo, H. Y.; Chen, Y.; Li, R. Y.; Zhao, N.; Luo, J.; Da Silva, J. G. P.; Mücke, R.; Kaghazchi, P.; Guo, X. X.; Sun, X. L. Design of a mixed conductive garnet/Li interface for dendrite-free solid lithium metal batteries. Energy Environ. Sci. 2020, 13, 127–134.
Tikekar, M. D.; Choudhury, S.; Tu, Z. Y.; Archer, L. A. Design principles for electrolytes and interfaces for stable lithium-metal batteries. Nat. Energy 2016, 1, 16114.
Zhu, Y. Z.; He, X. F.; Mo, Y. F. Origin of outstanding stability in the lithium solid electrolyte materials: Insights from thermodynamic analyses based on first-principles calculations. ACS Appl. Mater. Interfaces 2015, 7, 23685–23693.
Tan, J.; Matz, J.; Dong, P.; Shen, J. F.; Ye, M. X. A growing appreciation for the role of LiF in the solid electrolyte interphase. Adv. Energy Mater. 2021, 11, 2100046.
Swamy, T.; Park, R.; Sheldon, B. W.; Rettenwander, D.; Porz, L.; Berendts, S.; Uecker, R.; Carter, W. C.; Chiang, Y. M. Lithium metal penetration induced by electrodeposition through solid electrolytes: Example in single-crystal Li6La3ZrTaO12 garnet. J. Electrochem. Soc. 2018, 165, A3648–A3655.
Fu, K.; Gong, Y. H.; Liu, B. Y.; Zhu, Y. Z.; Xu, S. M.; Yao, Y. G.; Luo, W.; Wang, C. W.; Lacey, S. D.; Dai, J. Q. et al. Toward garnet electrolyte-based Li metal batteries: An ultrathin, highly effective, artificial solid-state electrolyte/metallic Li interface. Sci. Adv. 2017, 3, e1601659.
Li, C. L.; Gu, L.; Maier, J. Enhancement of the Li conductivity in LiF by introducing glass/crystal interfaces. Adv. Funct. Mater. 2012, 22, 1145–1149.
Zhang, X. Q.; Chen, X.; Xu, R.; Cheng, X. B.; Peng, H. J.; Zhang, R.; Huang, J. Q.; Zhang, Q. Columnar lithium metal anodes. Angew. Chem., Int. Ed. 2017, 56, 14207–14211.
Fan, L.; Zhuang, H. L.; Gao, L. N.; Lu, Y. Y.; Archer, L. A. Regulating Li deposition at artificial solid electrolyte interphases. J. Mater. Chem. A 2017, 5, 3483–3492.
Fan, X. L.; Ji, X.; Han, F. D.; Yue, J.; Chen, J.; Chen, L.; Deng, T.; Jiang, J. J.; Wang, C. S. Fluorinated solid electrolyte interphase enables highly reversible solid-state Li metal battery. Sci. Adv. 2018, 4, eaau9245.
Hu, A. J.; Chen, W.; Du, X. C.; Hu, Y.; Lei, T. Y.; Wang, H. B.; Xue, L. X.; Li, Y. Y.; Sun, H.; Yan, Y. C. et al. An artificial hybrid interphase for an ultrahigh-rate and practical lithium metal anode. Energy Environ. Sci. 2021, 14, 4115–4124.
Pierret, R. F. Semiconductor Device Fundamentals; Addison-Wesley: Boston, 1996.
Yildirim, H.; Kinaci, A.; Chan, M. K. Y.; Greeley, J. P. First-principles analysis of defect thermodynamics and ion transport in inorganic SEI compounds: LiF and NaF. ACS Appl. Mater. Interfaces 2015, 7, 18985–18996.
Kao, K. C. Dielectric Phenomena in Solids; Elsevier Academic Press: San Diego, 2004.
Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775.
Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186.
Kresse, G.; Furthmüller, J. Efficiency of ab initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Matter. Sci. 1996, 6, 15–50.
Perdew, J. P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868.
Monkhorst, H. J.; Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192.
Liu, Z.; Qi, Y.; Lin, Y. X.; Chen, L.; Chen, L. Q. Interfacial study on solid electrolyte interphase at Li metal anode: Implication for Li dendrite growth. J. Electrochem. Soc. 2016, 163, A592–A598.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Nos. 52072323, 52172240, and 11874307), Natural Science Foundation of Jiangxi Province (No. 20192ACBL20048), Natural Science Foundation of Jiangsu Province (No. BK20200800), Scientific Research Project of Fujian Provincial Department of Education (No. JAT191150), the Fundamental Research Funds for the Central Universities (No. 20720200075), and the Double-First Class Foundation of Materials and Intelligent Manufacturing Discipline of Xiamen University.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Luo, L., Zheng, F., Gao, H. et al. Tuning the electron transport behavior at Li/LATP interface for enhanced cyclability of solid-state Li batteries. Nano Res. 16, 1634–1641 (2023). https://doi.org/10.1007/s12274-022-5136-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-5136-2