Abstract
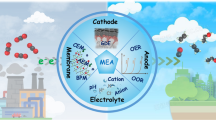
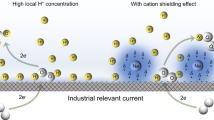
Membrane electrode assembly reactor offers great promise toward practical CO2 electrolysis. Unfortunately, traditional proton exchange membrane possesses strong acidic chemical environment, which facilitates undesired hydrogen evolution reaction. Here we report a proton antagonist strategy, through which the proton diffusion pathways have been severely impeded by Na+ cation to produce an alkaline-rich environment. With this new membrane electrode assembly, we can significantly suppress the hydrogen evolution and achieve a Faradaic efficiency of 95.7% for CO with 51.5% energy efficiency. In addition, our proton antagonist membrane outperforms the commercial anion exchange membrane in both conductivity and oxidation resistance lifetime, which are crucial for large scale electrolysis of carbon neutral chemicals.

Similar content being viewed by others
References
Li, Y. H.; Ozden, A.; Leow, W. R.; Ou, P. F.; Huang, J. E.; Wang, Y. H.; Bertens, K.; Xu, Y.; Liu, Y.; Roy, C. et al. Redox-mediated electrosynthesis of ethylene oxide from CO2 and water. Nat. Catal. 2022, 5, 185–192.
Xiao, T. S.; Tang, C.; Li, H. B.; Ye, T.; Ba, K.; Gong, P.; Sun, Z. Z. CO2 reduction with coin catalyst. Nano Res. 2022, 15, 3859–3865.
García de Arquer, F. P.; Dinh, C. T.; Ozden, A.; Wicks, J.; McCallum, C.; Kirmani Ahmad, R.; Nam, D. H.; Gabardo, C.; Seifitokaldani, A.; Wang, X. et al. CO2 electrolysis to multicarbon products at activities greater than 1 A·cm−2. Science 2020, 367, 661–666.
Wakerley, D.; Lamaison, S.; Wicks, J.; Clemens, A.; Feaster, J.; Corral, D.; Jaffer, S. A.; Sarkar, A.; Fontecave, M.; Duoss, E. B. et al. Gas diffusion electrodes, reactor designs and key metrics of low-temperature CO2 electrolysers. Nat. Energy 2022, 7, 130–143.
Millet, P.; Ngameni, R.; Grigoriev, S. A.; Mbemba, N.; Brisset, F.; Ranjbari, A.; Etiévant, C. PEM water electrolyzers: From electrocatalysis to stack development. Int. J. Hydrog. Energy 2010, 35, 5043–5052.
Salvatore, D. A.; Gabardo, C. M.; Reyes, A.; O’Brien, C. P.; Holdcroft, S.; Pintauro, P.; Bahar, B.; Hickner, M.; Bae, C.; Sinton, D. et al. Designing anion exchange membranes for CO2 electrolysers. Nat. Energy 2021, 6, 339–348.
Chen, N. J.; Paek, S. Y.; Lee, J. Y.; Park, J. H.; Lee, S. Y.; Lee, Y. M. High-performance anion exchange membrane water electrolyzers with a current density of 7.68 A·cm−2 and a durability of 1,000 hours. Energy Environ. Sci. 2021, 14, 6338–6348.
Chen, N. J.; Jin, Y. Q.; Liu, H. J.; Hu, C.; Wu, B.; Xu, S. Y.; Li, H.; Fan, J. T.; Lee, Y. M. Insight into the alkaline stability of N-heterocyclic ammonium groups for anion-exchange polyelectrolytes. Angew. Chem. 2021, 133, 19421–19429.
Banham, D.; Choi, J. Y.; Kishimoto, T.; Ye, S. Y. Integrating PGM-free catalysts into catalyst layers and proton exchange membrane fuel cell devices. Adv. Mater. 2019, 31, 1804846.
Kutz, R. B.; Chen, Q. M.; Yang, H. Z.; Sajjad, S. D.; Liu, Z. C.; Masel, I. R. Sustainion imidazolium-functionalized polymers for carbon dioxide electrolysis. Energy Technol. 2017, 5, 929–936.
Kim, Y.; Wang, Y. M.; France-Lanord, A.; Wang, Y. C.; Wu, Y. C. M.; Lin, S. B.; Li, Y. F.; Grossman, J. C.; Swager, T. M. Ionic highways from covalent assembly in highly conducting and stable anion exchange membrane fuel cells. J. Am. Chem. Soc. 2019, 141, 18152–18159.
Cha, M. S.; Park, J. E.; Kim, S.; Han, S. H.; Shin, S. H.; Yang, S. H.; Kim, T. H.; Yu, D. M.; So, S.; Hong, Y. T. et al. Poly (carbazole)-based anion-conducting materials with high performance and durability for energy conversion devices. Energy Environ. Sci. 2020, 13, 3633–3645.
Varcoe, J. R.; Atanassov, P.; Dekel, D. R.; Herring, A. M.; Hickner, M. A.; Kohl, P. A.; Kucernak, A. R.; Mustain, W. E.; Nijmeijer, K.; Scott, K. et al. Anion-exchange membranes in electrochemical energy systems. Energy Environ. Sci. 2014, 7, 3135–3191.
Fan, J. T.; Chen, M.; Zhao, Z. L.; Zhang, Z.; Ye, S. Y.; Xu, S. Y.; Wang, H. J.; Li, H. Bridging the gap between highly active oxygen reduction reaction catalysts and effective catalyst layers for proton exchange membrane fuel cells. Nat. Energy 2021, 6, 475–486.
Monteiro, M. C. O.; Philips, M. F.; Schouten, K. J. P.; Koper, M. T. M. Efficiency and selectivity of CO2 reduction to CO on gold gas diffusion electrodes in acidic media. Nat. Commun. 2021, 12, 4943.
Delacourt, C.; Ridgway, P. L.; Kerr, J. B.; Newman, J. Design of an electrochemical cell making syngas (CO+H2) from CO2 and H2O reduction at room temperature. Meet. Abstr. 2007, MA2007-02, 197.
Ma, L.; Fan, S.; Zhen, D. X.; Wu, X. M.; Liu, S. S.; Lin, J. J.; Huang, S. Q.; Chen, W.; He, G. H. Electrochemical reduction of CO2 in proton exchange membrane reactor: The function of buffer layer. Ind. Eng. Chem. Res. 2017, 56, 10242–10250.
Fujinuma, N.; Ikoma, A.; Lofland, S. E. Highly efficient electrochemical CO2 reduction reaction to CO with one-pot synthesized co-pyridine-derived catalyst incorporated in a Nafion-based membrane electrode assembly. Adv. Energy Mater. 2020, 10, 2001645.
O’Brien, C. P.; Miao, R. K.; Liu, S. J.; Xu, Y.; Lee, G.; Robb, A.; Huang, J. E.; Xie, K.; Bertens, K.; Gabardo, C. M. et al. Single pass CO2 conversion exceeding 85% in the electrosynthesis of multicarbon products via local CO2 regeneration. ACS Energy Lett. 2021, 6, 2952–2959.
Huang, J. E.; Li, F. W.; Ozden, A.; Sedighian Rasouli, A.; García de Arquer, F. P.; Liu, S. J.; Zhang, S. Z.; Luo, M. C.; Wang, X.; Lum, Y. et al. CO2 electrolysis to multicarbon products in strong acid. Science 2021, 372, 1074–1078.
Savage, J.; Tse, Y. L. S.; Voth, G. A. Proton transport mechanism of perfluorosulfonic acid membranes. J. Phys. Chem. C 2014, 118, 17436–17445.
Hu, J.; Zhang, H. M.; Xu, W. B.; Yuan, Z. Z.; Li, X. F. Mechanism and transfer behavior of ions in Nafion membranes under alkaline media. J. Membr. Sci. 2018, 566, 8–14.
Singh, R. K.; Kunimatsu, K.; Miyatake, K.; Tsuneda, T. Experimental and theoretical infrared spectroscopic study on hydrated Nafion membrane. Macromolecules 2016, 49, 6621–6629.
Jin, F.; Li, Y. D. A FTIR and TPD examination of the distributive properties of acid sites on ZSM-5 zeolite with pyridine as a probe molecule. Catal. Today 2009, 145, 101–107.
Liang, Z. X.; Chen, W. M.; Liu, J. G.; Wang, S. L.; Zhou, Z. H.; Li, W. Z.; Sun, G. Q.; Xin, Q. FT-IR study of the microstructure of Nafion® membrane. J. Membr. Sci. 2004, 233, 39–44.
Huang, W. W.; Frech, R.; Wheeler, R. A. Molecular structures and normal vibrations of CF3SO3− and its lithium ion pairs and aggregates. J. Phys. Chem. 1994, 98, 100–110.
Han, J. H.; Lee, K. W.; Jeon, G. W.; Lee, C. E.; Park, W. K.; Choi, E. H. 1H nuclear magnetic resonance study of hydrated water dynamics in perfluorosulfonic acid ionomer Nafion. Appl. Phys. Lett. 2015, 106, 023104.
Ye, G.; Janzen, N.; Goward, G. R. Solid-state NMR study of two classic proton conducting polymers: Nafion and sulfonated poly(ether ether ketone)s. Macromolecules 2006, 39, 3283–3290.
Friedman, A. K.; Shi, W. Q.; Losovyj, Y.; Siedle, A. R.; Baker, L. A. Mapping microscale chemical heterogeneity in Nafion membranes with X-ray photoelectron spectroscopy. J. Electrochem. Soc. 2018, 165, H733–H741.
Lettenmeier, P.; Wang, L.; Golla-Schindler, U.; Gazdzicki, P.; Cañas, N. A.; Handl, M.; Hiesgen, R.; Hosseiny, S. S.; Gago, A. S.; Friedrich, K. A. Nanosized IrOx-Ir catalyst with relevant activity for anodes of proton exchange membrane electrolysis produced by a cost-effective procedure. Angew. Chem. 2016, 128, 752–756.
Choi, Y.; Ryu, G. H.; Min, S. H.; Lee, B. R.; Song, M. H.; Lee, Z.; Kim, B. S. Interface-controlled synthesis of heterodimeric silver-carbon nanoparticles derived from polysaccharides. ACS Nano 2014, 8, 11377–11385.
Han, A. D.; Fu, C. H.; Yan, X. H.; Chen, J. R.; Cheng, X. J.; Ke, C. C.; Hou, J. B.; Shen, S. Y.; Zhang, J. L. Effect of cobalt ion contamination on proton conduction of ultrathin Nafion film. Int. J. Hydrog. Energy 2020, 45, 25276–25285.
Zhang, X.; Zhao, X. H.; Zhu, P.; Adler, Z.; Wu, Z. Y.; Liu, Y. Y.; Wang, H. T. Electrochemical oxygen reduction to hydrogen peroxide at practical rates in strong acidic media. Nat. Commun. 2022, 13, 2880.
Yang, K. L.; Li, M. R.; Subramanian, S.; Blommaert, M. A.; Smith, W. A.; Burdyny, T. Cation-driven increases of CO2 utilization in a bipolar membrane electrode assembly for CO2 electrolysis. ACS Energy Lett. 2021, 6, 4291–4298.
Wang, X.; Wang, Z. Y.; García de Arquer, F. P.; Dinh, C. T.; Ozden, A.; Li, Y. C.; Nam, D. H.; Li, J.; Liu, Y. S.; Wicks, J. et al. Efficient electrically powered CO2-to-ethanol via suppression of deoxygenation. Nat. Energy 2020, 5, 478–486.
Verma, S.; Lu, X.; Ma, S. C.; Masel, R. I.; Kenis, P. J. A. The effect of electrolyte composition on the electroreduction of CO2 to CO on Ag based gas diffusion electrodes. Phys. Chem. Chem. Phys. 2016, 18, 7075–7084.
Verma, S.; Hamasaki, Y.; Kim, C.; Huang, W. X.; Lu, S.; Jhong, H. R. M.; Gewirth, A. A.; Fujigaya, T.; Nakashima, N.; Kenis, P. J. Insights into the low overpotential electroreduction of CO2 to CO on a supported gold catalyst in an alkaline flow electrolyzer. ACS Energy Lett. 2018, 3, 193–198.
Zheng, T. T.; Jiang, K.; Ta, N.; Hu, Y. F.; Zeng, J.; Liu, J. Y.; Wang, H. T. Large-scale and highly selective CO2 electrocatalytic reduction on nickel single-atom catalyst. Joule 2019, 3, 265–278.
Xia, C.; Qiu, Y. R.; Xia, Y.; Zhu, P.; King, G.; Zhang, X.; Wu, Z. Y.; Kim, J. Y.; Cullen, D. A.; Zheng, D. X. et al. General synthesis of single-atom catalysts with high metal loading using graphene quantum dots. Nat. Chem. 2021, 13, 887–894.
Jhong, H. R. M.; Tornow, C. E.; Kim, C.; Verma, S.; Oberst, J. L.; Anderson, P. S.; Gewirth, A. A.; Fujigaya, T.; Nakashima, N.; Kenis, P. J. Gold nanoparticles on polymer-wrapped carbon nanotubes: An efficient and selective catalyst for the electroreduction of CO2. ChemPhysChem 2017, 18, 3274–3279.
Ma, S. C.; Lan, Y. C.; Perez, G. M. J.; Moniri, S.; Kenis, P. J. A. Silver supported on titania as an active catalyst for electrochemical carbon dioxide reduction. ChemSusChem 2014, 7, 866–874.
Ma, S. C.; Liu, J. F.; Sasaki, K.; Lyth, S. M.; Kenis, P. J. A. Carbon foam decorated with silver nanoparticles for electrochemical CO2 conversion. Energy Technol. 2017, 5, 861–863.
Tornow, C. E.; Thorson, M. R.; Ma, S. C.; Gewirth, A. A.; Kenis, P. J. A. Nitrogen-based catalysts for the electrochemical reduction of CO2 to CO. J. Am. Chem. Soc. 2012, 134, 19520–19523.
Frühwirt, P.; Kregar, A.; Törring, J. T.; Katrašnik, T.; Gescheidt, G. Holistic approach to chemical degradation of Nafion membranes in fuel cells: Modelling and predictions. Phys. Chem. Chem. Phys. 2020, 22, 5647–5666.
Chen, C. B.; Li, Y. F.; Yang, P. D. Address the “alkalinity problem” in CO2 electrolysis with catalyst design and translation. Joule 2021, 5, 737–742.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 21771040, 62074043, and 11705152), the National Key Research and Development Program of China (No. 2017YFA0207303), the Yiwu Research Institute Program of Fudan University (20-1-04).
Author information
Authors and Affiliations
Contributions
T.X. and Z.S. conceived the project, analyzed the data and wrote the paper. T.X. prepared all the catalysts. T.X., X.Y and Y.M. performed the CO2RR including the MEA evaluation and analyzed the gas products. T.X. and S.Z. contributed to the AEM stability tests, and characterizations of the material. T.X. T.Y and H. L. contributed to the supporting method and experiments. Z.S. supervised all research phases and revised the manuscript. All authors read and commented on the manuscript.
Corresponding author
Additional information
Conflict of interest
All authors declare no competing interests.
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Xiao, T., Ma, Y., Zeng, S. et al. Proton antagonist membrane towards exclusive CO2 reduction. Nano Res. 16, 4589–4595 (2023). https://doi.org/10.1007/s12274-022-5067-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-5067-y