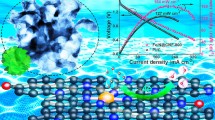
Abstract
The development of electrocatalysts toward the hydrogen evolution reaction (HER) with high-current-density capability is critical for the practical application of water splitting for hydrogen production. While Pt-based materials are regarded as the most efficient HER catalysts, they suffer from scarcity and high price. Thus, it is of vital importance to lower the loading of Pt while maintaining high activity. Here, we report the fabrication of a monolithic aligned porous carbon film electrode co-modified with Pt single atoms and Pt nanoclusters (Pt SA/NC-AF) containing ultralow Pt content (0.038 wt.%) via a facile electrochemical deposition process. Benefiting from the aligned porous structure of the carbon film and the high exposure of the Pt species, the optimized Pt SA/NC-AF electrode exhibits outstanding HER performance in 0.5 M H2SO4 with exceptional intrinsic activity (turnover frequency (TOF) = 904.9 s−1 at η = 100 mV) and ultrahigh mass activity (888.6 A·mgPt−1 at · = 100 mV). Further, it can deliver an industrially relevant current density of 1,000 mA·cm−2 at an overpotential as low as 139 mV. This work provides a feasible avenue for the rational design of metal single-atom and nanocluster catalysts and additionally promotes the application of ultralow-loading noble metal-based catalysts in high-rate hydrogen production.

Similar content being viewed by others
References
Xu, Y. L.; Wang, C.; Huang, Y. H.; Fu, J. Recent advances in electrocatalysts for neutral and large-current-density water electrolysis. Nano Energy 2021, 80, 105545.
Yang, Q.; Li, G. W.; Manna, K.; Fan, F. R.; Felser, C.; Sun, Y. Topological engineering of Pt-group-metal-based chiral crystals toward high-efficiency hydrogen evolution catalysts. Adv. Mater. 2020, 32, 1908518.
Jing, H. Y.; Zhu, P.; Zheng, X. B.; Zhang, Z. D.; Wang, D. S.; Li, Y. D. Theory-oriented screening and discovery of advanced energy transformation materials in electrocatalysis. Adv. Powder Mater. 2022, 1, 100013.
Zhu, J.; Hu, L. S.; Zhao, P. X.; Lee, L. Y. S.; Wong, K. Y. Recent advances in electrocatalytic hydrogen evolution using nanoparticles. Chem. Rev. 2020, 120, 851–918.
Luo, Y. T.; Zhang, Z. Y.; Chhowalla, M.; Liu, B. L. Recent advances in design of electrocatalysts for high-current-density water splitting. Adv. Mater. 2022, 34, 2108133.
Yu, Q. M.; Zhang, Z. Y.; Qiu, S. Y.; Luo, Y. T.; Liu, Z. B.; Yang, F. N.; Liu, H. M.; Ge, S. Y.; Zou, X. L.; Ding, B. F. et al. A Ta-TaS2 monolith catalyst with robust and metallic interface for superior hydrogen evolution. Nat. Commun. 2021, 12, 6051.
Nie, N. Z.; Zhang, D.; Wang, Z. C.; Qin, Y. N.; Zhai, X. J.; Yang, B.; Lai, J. P.; Wang, L. Superfast synthesis of densely packed and ultrafine Pt-lanthanide@KB via solvent-free microwave as efficient hydrogen evolution electrocatalysts. Small 2021, 17, 2102879.
Zhang, C. X.; Cui, Y. N.; Yang, Y. L.; Lu, L. G.; Yu, S. S.; Meng, Z. S.; Wu, Y. X.; Li, Y. X.; Wang, Y. A.; Tian, H. W. et al. Highly conductive amorphous pentlandite anchored with ultrafine platinum nanoparticles for efficient pH-universal hydrogen evolution reaction. Adv. Funct. Mater. 2021, 31, 2105372.
Zhang, F. F.; Zhu, Y. L.; Lin, Q.; Zhang, L.; Zhang, X. W.; Wang, H. T. Noble-metal single-atoms in thermocatalysis, electrocatalysis, and photocatalysis. Energy Environ. Sci. 2021, 14, 2954–3009.
Fei, H. L.; Dong, J. C.; Chen, D. L.; Hu, T. D.; Duan, X. D.; Shakir, I.; Huang, Y.; Duan, X. F. Single atom electrocatalysts supported on graphene or graphene-like carbons. Chem. Soc. Rev. 2019, 48, 5207–5241.
Pan, Y.; Zhang, C.; Liu, Z.; Chen, C.; Li, Y. D. Structural regulation with atomic-level precision: From single-atomic site to diatomic and atomic interface catalysis. Matter 2020, 2, 78–110.
Zhu, P.; Xiong, X.; Wang, D. S. Regulations of active moiety in single atom catalysts for electrochemical hydrogen evolution reaction. Nano Res. 2022, 15, 5792–5815.
Zheng, X. B.; Li, B. B.; Wang, Q. S.; Wang, D. S.; Li, Y. D. Emerging low-nuclearity supported metal catalysts with atomic level precision for efficient heterogeneous catalysis. Nano Res., in press, https://doi.org/10.1007/s12274-022-4429-9.
Li, R. Z.; Wang, D. S. Understanding the structure-performance relationship of active sites at atomic scale. Nano Res., in press, https://doi.org/10.1007/s12274-022-4371-x.
Yang, J. R.; Li, W. H.; Tan, S. D.; Xu, K. N.; Wang, Y.; Wang, D. S.; Li, Y. D. The electronic metal-support interaction directing the design of single atomic site catalysts: Achieving high efficiency towards hydrogen evolution. Angew. Chem., Int. Ed. 2021, 60, 19085–19091.
Yang, J. R.; Li, W. H.; Xu, K. N.; Tan, S. D.; Wang, D. S.; Li, Y. D. Regulating the tip effect on single-atom and cluster catalysts: Forming reversible oxygen species with high efficiency in chlorine evolution reaction. Angew. Chem., Int. Ed. 2022, 134, e202200366.
Ao, X.; Zhang, W.; Li, Z. S.; Li, J. G.; Soule, L.; Huang, X.; Chiang, W. H.; Chen, H. M.; Wang, C. D.; Liu, M. L. et al. Markedly enhanced oxygen reduction activity of single-atom Fe catalysts via integration with Fe nanoclusters. ACS Nano 2019, 13, 11853–11862.
Ao, X.; Zhang, W.; Zhao, B. T.; Ding, Y.; Nam, G.; Soule, L.; Abdelhafiz, A.; Wang, C. D.; Liu, M. L. Atomically dispersed Fe-N-C decorated with Pt-alloy core-shell nanoparticles for improved activity and durability towards oxygen reduction. Energy Environ. Sci. 2020, 13, 3032–3040.
Nie, Z. F.; Zhang, L. L.; Ding, X.; Cong, M. Y.; Xu, F. F.; Ma, L. H.; Guo, M. X.; Li, M. Z.; Zhang, L. X. Catalytic kinetics regulation for enhanced electrochemical nitrogen oxidation by Ru-nanoclusters-coupled Mn3O4 catalysts decorated with atomically dispersed Ru Atoms. Adv. Mater. 2022, 34, 2108180.
Liu, P. G.; Huang, Z. X.; Gao, X. P.; Hong, X.; Zhu, J. F.; Wang, G. M.; Wu, Y. E.; Zeng, J.; Zheng, X. S. Synergy between palladium single atoms and nanoparticles via hydrogen spillover for enhancing CO2 photoreduction to CH4. Adv. Mater. 2022, 34, 2200057.
Hu, Q.; Li, G. M.; Huang, X. W.; Wang, Z. Y.; Yang, H. P.; Zhang, Q. L.; Liu, J. H.; He, C. X. Electronic structure engineering of single atomic Ru by Ru nanoparticles to enable enhanced activity for alkaline water reduction. J. Mater. Chem. A 2019, 7, 19531–19538.
Luo, W. H.; Wang, Y.; Luo, L. X.; Gong, S.; Wei, M. N.; Li, Y. X.; Gan, X. P.; Zhao, Y. Y.; Zhu, Z. H.; Li, Z. Single-atom and bimetallic nanoalloy supported on nanotubes as a bifunctional electrocatalyst for ultrahigh-current-density overall water splitting. ACS Catal. 2022, 12, 1167–1179.
Ji, J. P.; Zhang, Y. P.; Tang, L. B.; Liu, C. Y.; Gao, X. H.; Sun, M. H.; Zheng, J. C.; Ling, M.; Liang, C. D.; Lin, Z. Platinum single-atom and cluster anchored on functionalized MWCNTs with ultrahigh mass efficiency for electrocatalytic hydrogen evolution. Nano Energy 2019, 63, 103849.
Lei, C. J.; Wang, Y.; Hou, Y.; Liu, P.; Yang, J.; Zhang, T.; Zhuang, X. D.; Chen, M. W.; Yang, B.; Lei, L. C. et al. Efficient alkaline hydrogen evolution on atomically dispersed Ni-Nx species anchored porous carbon with embedded Ni nanoparticles by accelerating water dissociation kinetics. Energy Environ. Sci. 2019, 12, 149–156.
Ramesh, R.; Han, S.; Nandi, D. K.; Sawant, S. Y.; Kim, D. H.; Cheon, T.; Cho, M. H.; Harada, R.; Shigetomi, T.; Suzuki, K. et al. Ultralow loading (single-atom and clusters) of the Pt catalyst by atomic layer deposition using dimethyl ((3, 4-η) N, N -dimethyl-3-butene-1-amine-N) platinum (DDAP) on the high-surface-area substrate for hydrogen evolution reaction. Adv. Mater. Interfaces 2021, 8, 2001508.
Cheng, Q. Q.; Hu, C. G.; Wang, G. L.; Zou, Z. Q.; Yang, H.; Dai, L. M. Carbon-defect-driven electroless deposition of Pt atomic clusters for highly efficient hydrogen evolution. J. Am. Chem. Soc. 2020, 142, 5594–5601.
Li, L.; Zhang, G. W.; Wang, B.; Yang, T.; Yang, S. C. Electrochemical formation of PtRu bimetallic nanoparticles for highly efficient and pH-universal hydrogen evolution reaction. J. Mater. Chem. A 2020, 8, 2090–2098.
Wang, Z. Y.; Yang, J.; Gan, J.; Chen, W. X.; Zhou, F. Y.; Zhou, X.; Yu, Z. Q.; Zhu, J. F.; Duan, X. Z.; Wu, Y. E. Electrochemical conversion of bulk platinum into platinum single-atom sites for the hydrogen evolution reaction. J. Mater. Chem. A 2020, 4, 10755–10760.
Li, Y.; Gu, Q. F.; Johannessen, B.; Zheng, Z.; Li, C.; Luo, Y. T.; Zhang, Z. Y.; Zhang, Q.; Fan, H. N.; Luo, W. B. et al. Synergistic Pt doping and phase conversion engineering in two-dimensional MoS2 for efficient hydrogen evolution. Nano Energy 2021, 84, 105898.
Nairan, A.; Liang, C. W.; Chiang, S. W.; Wu, Y.; Zou, P. C.; Khan, U.; Liu, W. D.; Kang, F. Y.; Guo, S. J.; Wu, J. B. et al. Proton selective adsorption on Pt-Ni nano-thorn array electrodes for superior hydrogen evolution activity. Energy Environ. Sci. 2021, 14, 1594–1601.
Liu, L.; Wang, Y.; Zhao, Y. Z.; Wang, Y.; Zhang, Z. L.; Wu, T.; Qin, W. J.; Liu, S. J.; Jia, B. R.; Wu, H. Y. et al. Ultrahigh Pt-mass-activity hydrogen evolution catalyst electrodeposited from bulk Pt. Adv. Funct. Mater. 2022, 32, 2112207.
Liang, C. W.; Zou, P. C.; Nairan, A.; Zhang, Y. Q.; Liu, J. X.; Liu, K. W.; Hu, S. Y.; Kang, F. Y.; Fan, H. J.; Yang, C. Exceptional performance of hierarchical Ni-Fe oxyhydroxide@NiFe alloy nanowire array electrocatalysts for large current density water splitting. Energy Environ. Sci. 2020, 13, 86–95.
Guo, M. R.; Qayum, A.; Dong, S.; Jiao, X. L.; Chen, D. R.; Wang, T. In situ conversion of metal (Ni, Co or Fe) foams into metal sulfide (Ni3S2, Co9S8 or FeS) foams with surface grown N-doped carbon nanotube arrays as efficient superaerophobic electrocatalysts for overall water splitting. J. Mater. Chem. A 2020, 8, 9239–9247.
Xu, W. W.; Lu, Z. Y.; Sun, X. M.; Jiang, L.; Duan, X. Superwetting electrodes for gas-involving electrocatalysis. Acc. Chem. Res. 2018, 51, 1590–1598.
Sun, H. M.; Yan, Z. H.; Liu, F. M.; Xu, W. C.; Cheng, F. Y.; Chen, J. Self-supported transition-metal-based electrocatalysts for hydrogen and oxygen evolution. Adv. Mater. 2020, 32, 1806326.
Shan, X. Y.; Liu, J.; Mu, H. R.; Xiao, Y.; Mei, B. B.; Liu, W. G.; Lin, G.; Jiang, Z.; Wen, L. P.; Jiang, L. An engineered superhydrophilic/superaerophobic electrocatalyst composed of the supported CoMoSx chalcogel for overall water splitting. Angew. Chem., Int. Ed. 2020, 59, 1659–1665.
Luo, Y. T.; Zhang, Z. Y.; Yang, F. N.; Li, J.; Liu, Z. B.; Ren, W. C.; Zhang, S.; Liu, B. L. Stabilized hydroxide-mediated nickel-based electrocatalysts for high-current-density hydrogen evolution in alkaline media. Energy Environ. Sci. 2021, 14, 4610–4619.
Wu, D. L.; Chen, D.; Zhu, J. W.; Mu, S. C. Ultralow Ru incorporated amorphous cobalt-based oxides for high-current-density overall water splitting in alkaline and seawater media. Small 2021, 17, 2102777.
Liu, R.; Gong, Z. C.; Liu, J. B.; Dong, J. C.; Liao, J. W.; Liu, H.; Huang, H. K.; Liu, J. J.; Yan, M. M.; Huang, K. et al. Design of aligned porous carbon films with single-atom Co-N-C sites for high-current-density hydrogen generation. Adv. Mater. 2021, 33, 2103533.
Jiang, K.; Liu, B. Y.; Luo, M.; Ning, S. C.; Peng, M.; Zhao, Y.; Lu, Y. R.; Chan, T. S.; de Groot, F. M. F. et al. Single platinum atoms embedded in nanoporous cobalt selenide as electrocatalyst for accelerating hydrogen evolution reaction. Nat. Commun. 2019, 10, 1743.
Peng, Y. W.; Shan, C. S.; Wang, H. J.; Hong, L. Y.; Yao, S.; Wu, R. J.; Zhang, Z. M.; Lu, T. B. Polyoxometalate-derived ultrasmall Pt2W/WO3 heterostructure outperforms platinum for large-current-density H2 evolution. Adv. Energy Mater. 2019, 9, 1900597.
Zhang, Z. R.; Feng, C.; Liu, C. X.; Zuo, M.; Qin, L.; Yan, X. P.; Xing, Y. L.; Li, H. L.; Si, R.; Zhou, S. M. et al. Electrochemical deposition as a universal route for fabricating single-atom catalysts. Nat. Commun. 2020, 11, 1215.
Kuang, Y. D.; Chen, C. J.; Kirsch, D.; Hu, L. B. Thick electrode batteries: Principles, opportunities, and challenges. Adv. Energy Mater. 2019, 9, 1901457.
Zhao, S.; Zhang, H. B.; Luo, J. Q.; Wang, Q. W.; Xu, B.; Hong, S.; Yu, Z. Z. Highly electrically conductive three-dimensional Ti3C2Tx MXene/reduced graphene oxide hybrid aerogels with excellent electromagnetic interference shielding performances. ACS Nano 2018, 12, 11193–11202.
Pan, F. P.; Li, B. Y.; Sarnello, E.; Fei, Y. H.; Gang, Y.; Xiang, X. M.; Du, Z. C.; Zhang, P.; Wang, G. F.; Nguyen, H. T. et al. Atomically dispersed iron-nitrogen sites on hierarchically mesoporous carbon nanotube and graphene nanoribbon networks for CO2 reduction. ACS Nano 2020, 14, 5506–5516.
Dimiev, A. M.; Khannanov, A.; Vakhitov, I.; Kiiamov, A.; Shukhina, K.; Tour, J. M. Revisiting the mechanism of oxidative unzipping of multiwall carbon nanotubes to graphene nanoribbons. ACS Nano 2018, 12, 3985–3993.
Xia, W.; Tang, J.; Li, J. J.; Zhang, S. H.; Wu, K. C. W.; He, J. P.; Yamauchi, Y. Defect-rich graphene nanomesh produced by thermal exfoliation of metal-organic frameworks for the oxygen reduction reaction. Angee. Chem., Int. Ed. 2019, 58, 13354–13359.
He, Q.; Zhou, Y. Z.; Shou, H. W.; Wang, X. Y.; Zhang, P. J.; Xu, W. J.; Qiao, S. C.; Wu, C. Q.; Liu, H. J.; Liu, D. B. et al. Synergic reaction kinetics over adjacent ruthenium sites for superb hydrogen generation in alkaline media. Adv. Mater. 2022, 34, 2110604.
Li, K.; Li, Y.; Wang, Y. M.; Ge, J. J.; Liu, C. P.; Xing, W. Enhanced electrocatalytic performance for the hydrogen evolution reaction through surface enrichment of platinum nanoclusters alloying with ruthenium in situ embedded in carbon. Energy Environ. Sci. 2018, 11, 1232–1239.
Zhang, D.; Wang, Z. C.; Wu, X. K.; Shi, Y.; Nie, N. Z.; Zhao, H.; Miao, H. F.; Chen, X. L.; Li, S. X.; Lai, J. P. et al. Noble metal (Pt, Rh, Pd, Ir) doped Ru/CNT ultra-small alloy for acidic hydrogen evolution at high current density. Small 2022, 18, 2104559.
Li, H. Y.; Chen, S. M.; Zhang, Y.; Zhang, Q. H.; Jia, X. F.; Zhang, Q.; Gu, L.; Sun, X. M.; Song, L.; Wang, X. Systematic design of superaerophobic nanotube-array electrode comprised of transition-metal sulfides for overall water splitting. Nat. Commun. 2018, 9, 2452.
Acknowledgements
H. F. acknowledges financial support from the National Natural Science Foundation of China (Nos. 51902099 and 92163116), Functamental Research Functs for the Central Universities (No. 531119200087), and the Innovative Research Groups of Hunan Province (No. 2020JJ1001). G. Y. acknowledges support from the Hunan Province Natural Science Foundation (No. 2020JJ4204).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2022_4749_MOESM1_ESM.pdf
Aligned porous carbon film with ultralow loadings of Pt single atoms and clusters for high-current-density hydrogen generation
Rights and permissions
About this article
Cite this article
Liu, R., Gong, Z., Yan, M. et al. Aligned porous carbon film with ultralow loadings of Pt single atoms and clusters for high-current-density hydrogen generation. Nano Res. 16, 256–263 (2023). https://doi.org/10.1007/s12274-022-4749-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-4749-9