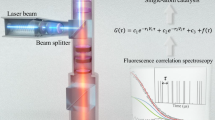
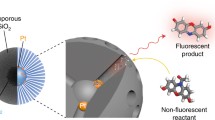
Abstract
Nanoparticles (NPs) play a vital role in the energy catalysis process, so understanding the heterogeneous catalytic properties of nanocatalysts is of great significance for rationally guiding the design of catalysts. However, the traditional method obtains the average information based on the whole and cannot study the catalytic activity of a single nanoparticle. It is critical to investigate the catalytic activity of individual nanoparticles using in situ techniques. This review summarizes some of Prof. Xu’s recent accomplishments in studying the catalytic behavior of nanoparticles at the single-particle level using single-molecule fluorescence microscopy (SMFM). These achievements include revealing the effect of size, shape, and surface atoms of Pd nanoparticles on catalytic kinetics and dynamics as well as obtaining the activation energy of single Au nanoparticles for catalytic reactions by single-molecule methods. It is the first time to study the kinetics and dynamics of single-atom Pt catalysts. Furthermore, the method was extended to study the Pt deactivation process for hydrogen oxidation reactions as well as the catalytic kinetics of two-electron oxygen reduction reactions of individual Fe3O4 nanoparticles in electrocatalysis. Finally, single-molecule super-resolution techniques were used to observe the evolution of the activity of single Sb doped TiO2 nanorod domains. These studies are of guiding significance for in-depth understanding and realization of rational design of optimal catalysts.

Similar content being viewed by others
References
Ma, Z. M.; Liu, S. Q.; Tang, N. F.; Song, T.; Motokura, K.; Shen, Z. M.; Yang, Y. Coexistence of Fe nanoclusters boosting Fe single atoms to generate singlet oxygen for efficient aerobic oxidation of primary amines to imines. ACS Catal. 2022, 12, 5595–5604.
Ryoo, R.; Kim, J.; Jo, C.; Han, S. W.; Kim, J. C.; Park, H.; Han, J.; Shin, H. S.; Shin, J. W. Rare-earth-platinum alloy nanoparticles in mesoporous zeolite for catalysis. Nature 2020, 585, 221–224.
Yao, Y. G.; Dong, Q.; Brozena, A.; Luo, J.; Miao, J. W.; Chi, M. F.; Wang, C.; Kevrekidis, I. G.; Ren, Z. J.; Greeley, J. et al. High-entropy nanoparticles: Synthesis-structure-property relationships and data-driven discovery. Science 2022, 376, eabn3103.
Liu, K. L.; Qin, R. X.; Zheng, N. F. Insights into the interfacial effects in heterogeneous metal nanocatalysts toward selective hydrogenation. J. Am. Chem. Soc. 2021, 143, 4483–4499.
Song, Y.; Ozdemir, E.; Ramesh, S.; Adishev, A.; Subramanian, S.; Harale, A.; Albuali, M.; Fadhel, B. A.; Jamal, A.; Moon, D. et al. Dry reforming of methane by stable Ni-Mo nanocatalysts on single-crystalline MgO. Science 2020, 367, 777–781.
Wang, H. W.; Gu, X. K.; Zheng, X. S.; Pan, H. B.; Zhu, J. F.; Chen, S.; Cao, L. N.; Li, W. X.; Lu, J. L. Disentangling the size-dependent geometric and electronic effects of palladium nanocatalysts beyond selectivity. Sci. Adv. 2019, 5, eaat6413.
Gao, D. F.; Zhou, H.; Wang, J.; Miao, S.; Yang, F.; Wang, G. X.; Wang, J. G.; Bao, X. H. Size-dependent electrocatalytic reduction of CO2 over Pd nanoparticles. J. Am. Chem. Soc. 2015, 137, 4288–4291.
Laletina, S. S.; Mamatkulov, M.; Shor, E. A.; Kaichev, V. V.; Genest, A.; Yudanov, I. V.; Rösch, N. Size-dependence of the adsorption energy of CO on Pt nanoparticles: Tracing two intersecting trends by DFT calculations. J. Phys. Chem. C 2017, 121, 17371–17377.
Li, J. B.; Qian, H. F.; Chen, H. L.; Zhao, Z.; Yuan, K. J.; Chen, G. X.; Miranda, A.; Guo, X. M.; Chen, Y. J.; Zheng, N. F. et al. Two distinctive energy migration pathways of monolayer molecules on metal nanoparticle surfaces. Nat. Commun. 2016, 7, 10749.
Yudanov, I. V.; Genest, A.; Schauermann, S.; Freund, H. J.; Rösch, N. Size dependence of the adsorption energy of CO on metal nanoparticles: A DFT search for the minimum value. Nano Lett. 2012, 12, 2134–2139.
Chen, T.; Tong, F. X.; Enderlein, J.; Zheng, Z. K. Plasmon-driven modulation of reaction pathways of individual Pt-modified Au nanorods. Nano Lett. 2020, 20, 3326–3330.
Tong, F. X.; Liang, X. Z.; Ma, F. H.; Bao, X. L.; Wang, Z. Y.; Liu, Y. Y.; Wang, P.; Cheng, H. F.; Dai, Y.; Huang, B. B. et al. Plasmon-mediated nitrobenzene hydrogenation with formate as the hydrogen donor studied at a single-particle level. ACS Catal. 2021, 11, 3801–3809.
Zheng, Z. K.; Tachikawa, T.; Majima, T. Single-particle study of Pt-modified Au nanorods for plasmon-enhanced hydrogen generation in visible to near-infrared region. J. Am. Chem. Soc. 2014, 136, 6870–6873.
Lin, M. H.; Zhou, Y. K.; Bu, L. Z.; Bai, C.; Tariq, M.; Wang, H. H.; Han, J. L.; Huang, X. Q.; Zhou, X. C. Single-nanoparticle coulometry method with high sensitivity and high throughput to study the electrochemical activity and oscillation of single nanocatalysts. Small 2021, 17, 2007302.
Liu, S. C.; Ying, Y. L.; Long, Y. T. Rapid ultrasensitive monitoring the single-particle surface-enhanced Raman scattering (SERS) using a dark-field microspectroscopy assisted system. Chin. Chem. Lett. 2020, 31, 473–475.
Chen, P.; Zhou, X. C.; Andoy, N. M.; Han, K. S.; Choudhary, E.; Zou, N. M.; Chen, G. Q.; Shen, H. Spatiotemporal catalytic dynamics within single nanocatalysts revealed by single-molecule microscopy. Chem. Soc. Rev. 2014, 43, 1107–1117.
Janssen, K. P. F.; De Cremer, G.; Neely, R. K.; Kubarev, A. V.; Van Loon, J.; Martens, J. A.; De Vos, D. E.; Roeffaers, M. B. J.; Hofkens, J. Single molecule methods for the study of catalysis: From enzymes to heterogeneous catalysts. Chem. Soc. Rev. 2014, 43, 990–1006.
Xu, W. L.; Shen, H.; Kim, Y. J.; Zhou, X. C.; Liu, G. K.; Park, J.; Chen, P. Single-molecule electrocatalysis by single-walled carbon nanotubes. Nano Lett. 2009, 9, 3968–3973.
Zou, N. M.; Zhou, X. C.; Chen, G. Q.; Andoy, N. M.; Jung, W.; Liu, G. K.; Chen, P. Cooperative communication within and between single nanocatalysts. Nat. Chem. 2018, 10, 607–614.
Lu, H. P.; Xun, L. Y.; Xie, X. S. Single-molecule enzymatic dynamics. Science 1998, 282, 1877–1882.
Roeffaers, M. B. J.; Sels, B. F.; Uji-I, H.; De Schryver, F. C.; Jacobs, P. A.; De Vos, D. E.; Hofkens, J. Spatially resolved observation of crystal-face-dependent catalysis by single turnover counting. Nature 2006, 439, 572–575.
Xu, W. L.; Kong, J. S.; Yeh, Y. T. E.; Chen, P. Single-molecule nanocatalysis reveals heterogeneous reaction pathways and catalytic dynamics. Nat. Mater. 2008, 7, 992–996.
Tachikawa, T.; Yamashita, S.; Majima, T. Evidence for crystal-face-dependent TiO2 photocatalysis from single-molecule imaging and kinetic analysis. J. Am. Chem. Soc. 2011, 133, 7197–7204.
Chen, T.; Dong, B.; Chen, K. C.; Zhao, F.; Cheng, X. D.; Ma, C. B.; Lee, S.; Zhang, P.; Kang, S. H.; Ha, J. W. et al. Optical superresolution imaging of surface reactions. Chem. Rev. 2017, 117, 7510–7537.
Dong, B.; Mansour, N.; Pei, Y. C.; Wang, Z. R.; Huang, T. X.; Filbrun, S. L.; Chen, M. D.; Cheng, X. D.; Pruski, M.; Huang, W. Y. et al. Single molecule investigation of nanoconfinement hydrophobicity in heterogeneous catalysis. J. Am. Chem. Soc. 2020, 142, 13305–13309.
Dong, B.; Pei, Y.; Zhao, F.; Goh, T. W.; Qi, Z.; Xiao, C.; Chen, K.; Huang, W.; Fang, N. In situ quantitative single-molecule study of dynamic catalytic processes in nanoconfinement. Nat. Catal. 2018, 1, 135–140.
Liu, X. D.; Chen, T.; Song, P.; Zhang, Y. W.; Xu, W. L. Single-molecule nanocatalysis of Pt nanoparticles. J. Phys. Chem. C 2018, 122, 1746–1752.
Sambur, J. B.; Chen, P. Approaches to single-nanoparticle catalysis. Annu. Rev. Phys. Chem. 2014, 65, 395–422.
Hu, S. L.; Li, W. X. Sabatier principle of metal-support interaction for design of ultrastable metal nanocatalysts. Science 2021, 374, 1360–1365.
Li, L. L.; Jiang, Y. F.; Zhang, T. H.; Cai, H. F.; Zhou, Y. L.; Lin, B. Y.; Lin, X. Y.; Zheng, Y.; Zheng, L. R.; Wang, X. Y. et al. Size sensitivity of supported Ru catalysts for ammonia synthesis: From nanoparticles to subnanometric clusters and atomic clusters. Chem 2022, 8, 749–768.
Zhan, W. C.; Shu, Y.; Sheng, Y. J.; Zhu, H. Y.; Guo, Y. L.; Wang, L.; Guo, Y.; Zhang, J. S.; Lu, G. Z.; Dai, S. Surfactant-assisted stabilization of Au colloids on solids for heterogeneous catalysis. Angew, Chem., Int. Ed. 2017, 56, 4494–4498.
Chen, T.; Zhang, Y. W.; Xu, W. L. Size-dependent catalytic kinetics and dynamics of Pd nanocubes: A single-particle study. Phys. Chem. Chem. Phys. 2016, 18, 22494–22502.
Chen, T.; Chen, S.; Zhang, Y. W.; Qi, Y. F.; Zhao, Y. Z.; Xu, W. L.; Zeng, J. Catalytic kinetics of different types of surface atoms on shaped Pd nanocrystals. Angew, Chem., Int. Ed. 2016, 55, 1839–1843.
Tarnev, T.; Aiyappa, H. B.; Botz, A.; Erichsen, T.; Ernst, A.; Andronescu, C.; Schuhmann, W. Scanning electrochemical cell microscopy investigation of single ZIF-derived nanocomposite particles as electrocatalysts for oxygen evolution in alkaline media. Angew. Chem., Int. Ed. 2019, 58, 14265–14269.
Ustarroz, J.; Ornelas, I. M.; Zhang, G. H.; Perry, D.; Kang, M.; Bentley, C. L.; Walker, M.; Unwin, P. R. Mobility and poisoning of mass-selected platinum nanoclusters during the oxygen reduction reaction. ACS Catal. 2018, 8, 6775–6790.
Chen, G. Y.; Song, X. Y.; Richardson, T. J. Electron microscopy study of the LiFePO4 to FePO4 phase transition. Electrochem. Solid State Lett. 2006, 9, A295–A298.
Zhang, J. Y.; Qian, J. M.; Ran, J. Q.; Xi, P. X.; Yang, L. J.; Gao, D. Q. Engineering lower coordination atoms onto NiO/Co3O4 heterointerfaces for boosting oxygen evolution reactions. ACS Catal. 2020, 10, 12376–12384.
Crespo-Quesada, M.; Yarulin, A.; Jin, M. S.; Xia, Y. N.; Kiwi-Minsker, L. Structure sensitivity of alkynol hydrogenation on shape- and size-controlled palladium nanocrystals: Which sites are most active and selective? J. Am. Chem. Soc. 2011, 133, 12787–12794.
Van Hardeveld, R; Hartog, F. Statistics of surface atoms and surface sites on metal crystals. Surf. Sci. 1969, 15, 189–230.
Kaatz, F. H.; Bultheel, A. Catalytic thermodynamic model for nanocluster adsorbates. Catal. Today 2021, 360, 157–164.
Kanduč, M.; Kim, W. K.; Roa, R.; Dzubiella, J. How the shape and chemistry of molecular penetrants control responsive hydrogel permeability. ACS Nano 2021, 15, 614–624.
Narayanan, R.; El-Sayed, M. A. Catalysis with transition metal nanoparticles in colloidal solution: Nanoparticle shape dependence and stability. J. Phys. Chem. B 2005, 109, 12663–12676.
Laskar, M.; Skrabalak, S. E. Decoupling the geometric parameters of shape-controlled Pd nanocatalysts. ACS Catal. 2014, 4, 1120–1128.
Li, G. Q.; Kobayashi, H.; Dekura, S.; Ikeda, R.; Kubota, Y.; Kato, K.; Takata, M.; Yamamoto, T.; Matsumura, S.; Kitagawa, H. Shape-dependent hydrogen-storage properties in Pd nanocrystals: Which does hydrogen prefer, octahedron (111) or cube (100)? J. Am. Chem. Soc. 2014, 136, 10222–10225.
Chen, T.; Chen, S.; Song, P.; Zhang, Y. W.; Su, H. Y.; Xu, W. L.; Zeng, J. Single-molecule nanocatalysis reveals facet-dependent catalytic kinetics and dynamics of pallidium nanoparticles. ACS Catal. 2017, 7, 2967–2972.
Li, S.; Chen, B. B.; Wang, Y.; Ye, M. Y.; Van Aken, P. A.; Cheng, C.; Thomas, A. Oxygen-evolving catalytic atoms on metal carbides. Nat. Mater. 2021, 20, 1240–1247.
Zhai, Y. P.; Pierre, D.; Si, R.; Deng, W. L.; Ferrin, P.; Nilekar, A. U.; Peng, G. W.; Herron, J. A.; Bell, D. C.; Saltsburg, H. et al. Alkali-stabilized Pt-OHx species catalyze low-temperature water-gas shift reactions. Science 2010, 329, 1633–1636.
Vilé, G.; Albani, D.; Nachtegaal, M.; Chen, Z. P.; Dontsova, D.; Antonietti, M.; López, N.; Pérez-Ramírez, J. A stable single-site palladium catalyst for hydrogenations. Angew, Chem., Int. Ed. 2015, 54, 11265–11269.
Poerwoprajitno, A. R.; Gloag, L.; Watt, J.; Cheong, S.; Tan, X.; Lei, H.; Tahini, H. A.; Henson, A.; Subhash, B.; Bedford, N. M. et al. A single-Pt-atom-on-Ru-nanoparticle electrocatalyst for CO-resilient methanol oxidation. Nat. Catal. 2022, 5, 231–237.
Wu, Y. Q.; Wu, Q.; Zhang, Q. Q.; Lou, Z. Z.; Liu, K. F.; Ma, Y. D.; Wang, Z. Y.; Zheng, Z. K.; Cheng, H. F.; Liu, Y. Y. et al. An organometal halide perovskite supported Pt single-atom photocatalyst for H2 evolution. Energy Environ. Sci. 2022, 15, 1271–1281.
Zhang, J. J.; Wang, E. Q.; Cui, S. Q.; Yang, S. B.; Zou, X. L.; Gong, Y. J. Single-atom Pt anchored on oxygen vacancy of monolayer Ti3C2Tx for superior hydrogen evolution. Nano Lett. 2022, 22, 1398–1405.
Zhang, L.; Wang, Q.; Li, L. L.; Banis, M. N.; Li, J. J.; Adair, K.; Sun, Y. P.; Li, R. Y.; Zhao, Z. J.; Gu, M. et al. Single atom surface engineering: A new strategy to boost electrochemical activities of Pt catalysts. Nano Energy 2022, 93, 106813.
Cao, Y. H.; Guo, L.; Dan, M.; Doronkin, D. E.; Han, C. Q.; Rao, Z. Q.; Liu, Y.; Meng, J.; Huang, Z. A.; Zheng, K. B. et al. Modulating electron density of vacancy site by single Au atom for effective CO2 photoreduction. Nat. Commun. 2021, 12, 1675.
Liu, H.; Grasseschi, D.; Dodda, A.; Fujisawa, K.; Olson, D.; Kahn, E.; Zhang, F.; Zhang, T. Y.; Lei, Y.; Branco, R. B. N. et al. Spontaneous chemical functionalization via coordination of Au single atoms on monolayer MoS2. Sci. Adv. 2020, 6, eabc93.
Sun, H.; Yin, H. Q.; Shi, W. X.; Yang, L. L.; Guo, X. W.; Lin, H.; Zhang, J. W.; Lu, T. B.; Zhang, Z. M. Porous β-FeOOH nanotube stabilizing Au single atom for high-efficiency nitrogen fixation. Nano Res. 2022, 15, 3026–3033.
Xi, W.; Wang, K.; Shen, Y. L.; Ge, M. K.; Deng, Z. L.; Zhao, Y. F.; Cao, Q. E.; Ding, Y.; Hu, G. Z.; Luo, J. Dynamic Co-catalysis of Au single atoms and nanoporous Au for methane pyrolysis. Nat. Commun. 2020, 11, 1919.
He, Q.; Qiao, S. C.; Zhou, Q.; Zhou, Y. Z.; Shou, H. W.; Zhang, P. J.; Xu, W.; Liu, D. B.; Chen, S. M.; Wu, X. J. et al. Confining high-valence iridium single sites onto nickel oxyhydroxide for robust oxygen evolution. Nano Lett. 2022, 22, 3832–3839.
Iemhoff, A.; Vennewald, M.; Artz, J.; Mebrahtu, C.; Meledin, A.; Weirich, T. E.; Hartmann, H.; Besmehn, A.; Aramini, M.; Venturini, F. et al. On the stability of isolated iridium sites in N-rich frameworks against agglomeration under reducing conditions. ChemCatChem 2022, 14, e202200179.
Liu, C. X.; Pan, G. H.; Liang, N. J.; Hong, S.; Ma, J. Y.; Liu, Y. Z. Ir single atom catalyst loaded on amorphous carbon materials with high HER activity. Adv. Sci. 2022, 9, 2105392.
Chen, W.; Wu, B. B.; Wang, Y. Y.; Zhou, W.; Li, Y. Y.; Liu, T. Y.; Xie, C.; Xu, L. T.; Du, S. Q.; Song, M. L. et al. Deciphering the alternating synergy between interlayer Pt single-atom and NiFe layered double hydroxide for overall water splitting. Energy Environ. Sci. 2021, 14, 6428–6440.
Jiang, D.; Yao, Y. G.; Li, T. Y.; Wan, G.; Pereira-Hernandez, X. I.; Lu, Y. B.; Tian, J. S.; Khivantsev, K.; Engelhard, M. H.; Sun, C. J. et al. Tailoring the local environment of platinum in single-atom Pt1/CeO2 catalysts for robust low-temperature CO oxidation. Angew, Chem., Int. Ed. 2021, 60, 26054–26062.
Lai, W. H.; Zhang, L. F.; Yan, Z. C.; Hua, W. B.; Indris, S.; Lei, Y. J.; Liu, H. W.; Wang, Y. X.; Hu, Z. P.; Liu, H. K. et al. Activating inert surface Pt single atoms via subsurface doping for oxygen reduction reaction. Nano Lett. 2021, 21, 7970–7978.
Liu, X. D.; Ge, X.; Cao, J.; Xiao, Y.; Wang, Y.; Zhang, W.; Song, P.; Xu, W. L. Revealing the catalytic kinetics and dynamics of individual Pt atoms at the single-molecule level. Proc. Natl. Acad. Sci. USA 2022, 119, e2114639119.
Grunes, J.; Zhu, J.; Anderson, E. A.; Somorjai, G. A. Ethylene hydrogenation over platinum nanoparticle array model catalysts fabricated by electron beam lithography: Determination of active metal surface area. J. Phys. Chem. B 2002, 106, 11463–11468.
Moseler, M.; Walter, M.; Yoon, B.; Landman, U.; Habibpour, V.; Harding, C.; Kunz, S.; Heiz, U. Oxidation state and symmetry of magnesia-supported Pd13Ox nanocatalysts influence activation barriers of CO oxidation. J. Am. Chem. Soc. 2012, 134, 7690–7699.
Chen, T.; Zhang, Y. W.; Xu, W. L. Single-molecule nanocatalysis reveals catalytic activation energy of single nanocatalysts. J. Am. Chem. Soc. 2016, 138, 12414–12421.
Völkening, S.; Bedürftig, K.; Jacobi, K.; Wintterlin, J.; Ertl, G. Dual-path mechanism for catalytic oxidation of hydrogen on platinum surfaces. Phys. Rev. Lett. 1999, 83, 2672–2675.
Yu, X. W.; Ye, S. Y. Recent advances in activity and durability enhancement of Pt/C catalytic cathode in PEMFC: Part II: Degradation mechanism and durability enhancement of carbon supported platinum catalyst. J. Power Sour. 2007, 172, 145–154.
Zhang, Y. W.; Chen, T.; Alia, S.; Pivovar, B. S.; Xu, W. L. Single-molecule nanocatalysis shows in situ deactivation of Pt/C electrocatalysts during the hydrogen-oxidation reaction. Angew, Chem., Int. Ed. 2016, 55, 3086–3090.
Jiang, Y. Y.; Ni, P. J.; Chen, C. X.; Lu, Y. Z.; Yang, P.; Kong, B.; Fisher, A.; Wang, X. Selective electrochemical H2O2 production through two-electron oxygen electrochemistry. Adv. Energy Mater. 2018, 8, 1801909.
Yamanaka, I.; Onizawa, T.; Takenaka, S.; Otsuka, K. Direct and continuous production of hydrogen peroxide with 93% selectivity using a fuel-cell system. Angew, Chem., Int. Ed. 2003, 42, 3653–3655.
Martínez-Huitle, C. A.; Ferro, S. Electrochemical oxidation of organic pollutants for the wastewater treatment: Direct and indirect processes. Chem. Soc. Rev. 2006, 35, 1324–1340.
Campos-Martin, J. M.; Blanco-Brieva, G.; Fierro, J. L. G. Hydrogen peroxide synthesis: An outlook beyond the anthraquinone process. Angew, Chem., Int. Ed. 2006, 45, 6962–6984.
Mounfield III, W. P.; Garg, A.; Shao-Horn, Y.; Roman-Leshkov, Y. Electrochemical oxygen reduction for the production of hydrogen peroxide. Chem 2018, 4, 18–19.
Jung, E.; Shin, H.; Lee, B. H.; Efremov, V.; Lee, S.; Lee, H. S.; Kim, J.; Hooch Antink, W.; Park, S.; Lee, K. S. et al. Atomic-level tuning of Co−N−C catalyst for high-performance electrochemical H2O2 production. Nat. Mater. 2020, 19, 436–442.
Wang, Y. H.; Pegis, M. L.; Mayer, J. M.; Stahl, S. S. Molecular cobalt catalysts for O2 reduction: Low-overpotential production of H2O2 and comparison with iron-based catalysts. J. Am. Chem. Soc. 2017, 139, 16458–16461.
Yang, S.; Kim, J.; Tak, Y. J.; Soon, A.; Lee, H. Single-atom catalyst of platinum supported on titanium nitride for selective electrochemical reactions. Angew, Chem., Int. Ed. 2016, 55, 2058–2062.
Lu, Y. Z.; Jiang, Y. Y.; Gao, X. H.; Wang, X. D.; Chen, W. Strongly coupled pd nanotetrahedron/tungsten oxide nanosheet hybrids with enhanced catalytic activity and stability as oxygen reduction electrocatalysts. J. Am. Chem. Soc. 2014, 136, 11687–11697.
Yang, S.; Verdaguer-Casadevall, A.; Arnarson, L.; Silvioli, L.; Čolić, V.; Frydendal, R.; Rossmeisl, J.; Chorkendorff, I.; Stephens, I. E. L. Toward the decentralized electrochemical production of H2O2: A focus on the catalysis. ACS Catal. 2018, 8, 4064–4081.
Xiao, Y.; Hong, J.; Wang, X.; Chen, T.; Hyeon, T.; Xu, W. L. Revealing kinetics of two-electron oxygen reduction reaction at single-molecule level. J. Am. Chem. Soc. 2020, 142, 13201–13209.
Liu, C. H.; Tseng, W. L. Oxidase-functionalized Fe3O4 nanoparticles for fluorescence sensing of specific substrate. Anal. Chim. Acta 2011, 703, 87–93.
Shashkova, S.; Leake, M. C. Single-molecule fluorescence microscopy review: Shedding new light on old problems. Biosci. Rep. 2017, 37, BSR20170031.
Wang, W. X.; Shen, H.; Moringo, N. A.; Carrejo, N. C.; Ye, F.; Robinson, J. T.; Landes, C. F. Super-temporal-resolved microscopy reveals multistep desorption kinetics of α-lactalbumin from nylon. Langmuir 2018, 34, 6697–6702.
Moringo, N. A.; Shen, H.; Bishop, L. D. C.; Wang, W. X.; Landes, C. F. Enhancing analytical separations using super-resolution microscopy. Annu. Rev. Phys. Chem. 2018, 69, 353–375.
Huang, B.; Wang, W. Q.; Bates, M.; Zhuang, X. W. Three-dimensional super-resolution imaging by stochastic optical reconstruction microscopy. Science 2008, 319, 810–813.
Zhang, Y. W.; Lucas, J. M.; Song, P.; Beberwyck, B.; Fu, Q.; Xu, W. L.; Alivisatos, A. P. Superresolution fluorescence mapping of single-nanoparticle catalysts reveals spatiotemporal variations in surface reactivity. Proc. Natl. Acad. Sci. USA 2015, 112, 8959–8964.
Zhou, X. C.; Andoy, N. M.; Liu, G. K.; Choudhary, E.; Han, K. S.; Shen, H.; Chen, P. Quantitative super-resolution imaging uncovers reactivity patterns on single nanocatalysts. Nat. Nanotechnol. 2012, 7, 237–241.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 21925205, 22072145, 21733004, and 21721003), the National Key Research and Development Program of China (Nos. 2017YFE9127900 and 2018YFB1502302), and K. C. Wong Education Foundation and Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cao, J., Zhang, D. & Xu, W. Recent progress in single-molecule fluorescence technology in nanocatalysis. Nano Res. 15, 10316–10327 (2022). https://doi.org/10.1007/s12274-022-4713-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-4713-8