Abstract
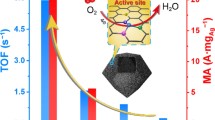
The electrosynthesis of hydrogen peroxide (H2O2) from oxygen reduction reaction (ORR) via a two-electron pathway provides an appealing alternative to the energy-intensive anthraquinone route; however, the development of ORR with high selectivity and durability for H2O2 production is still challenging. Herein, we demonstrate an active and stable catalyst, composing of highly dispersed Ag nanoclusters on N-doped hollow carbon spheres (NC-Ag/NHCS), which can effectively reduce O2 molecules into H2O2 with a selectivity of 89%–91% in a potential range from 0.2 to 0.7 V (vs. reversible hydrogen electrode (RHE)) in acidic media. Strikingly, NC-Ag/NHCS achieve a mass activity of 27.1 A·g−1 and a yield rate of 408 mmol·gcat.−1·h−1 at 0.7 V, both of which are comparable with the best-reported results. Furthermore, NC-Ag/NHCS enable catalyzing H2O2 production with a stable current density over 48-h electrolysis and only about 9.8% loss in selectivity after 10,000 cycles. Theoretical analyses indicate that Ag nanoclusters can contribute more electrons to favor the protonation of adsorbed O2, thus leading to a high H2O2 selectivity. This work confirms the great potential of metal nanocluster-based materials for H2O2 electrosynthesis under ambient conditions.

Similar content being viewed by others
References
Brillas, E.; Sirés, I.; Oturan, M. A. Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem. Rev. 2009, 109, 6570–6631.
Jung, E.; Shin, H.; Antink, W. H.; Sung, Y. E.; Hyeon, T. Recent advances in electrochemical oxygen reduction to H2O2: Catalyst and cell design. ACS Energy Lett. 2020, 5, 1881–1892.
Lu, Z. Y.; Chen, G. X.; Siahrostami, S.; Chen, Z. H.; Liu, K.; Xie, J.; Liao, L.; Wu, T.; Lin, D. C.; Liu, Y. Y. et al. High-efficiency oxygen reduction to hydrogen peroxide catalysed by oxidized carbon materials. Nat. Catal. 2018, 1, 156–162.
Shen, R. A.; Chen, W. X.; Peng, Q.; Lu, S. Q.; Zheng, L. R.; Cao, X.; Wang, Y.; Zhu, W.; Zhang, J. T.; Zhuang, Z. B. et al. High-concentration single atomic Pt sites on hollow CuSx for selective O2 reduction to H2O2 in acid solution. Chem 2019, 5, 2099–2110.
Jung, E.; Shin, H.; Lee, B. H.; Efremov, V.; Lee, S.; Lee, H. S.; Kim, J.; Antink, W. H.; Park, S.; Lee, K. S. et al. Atomic-level tuning of Co-N-C catalyst for high-performance electrochemical H2O2 production. Nat. Mater. 2020, 19, 436–442.
Dong, K.; Liang, J.; Wang, Y. Y.; Xu, Z. Q.; Liu, Q.; Luo, Y. L.; Li, T. S.; Li, L.; Shi, X. F.; Asiri, A. M. et al. Honeycomb carbon nanofibers: A superhydrophilic O2-entrapping electrocatalyst enables ultrahigh mass activity for the two-electron oxygen reduction reaction. Angew. Chem., Int. Ed. 2021, 60, 10583–10587.
Melchionna, M.; Fornasiero, P.; Prato, M. The rise of hydrogen peroxide as the main product by metal-free catalysis in oxygen reductions. Adv. Mater. 2019, 31, 1802920.
Li, X. G.; Tang, S. S.; Dou, S.; Fan, H. J.; Choksi, T. S.; Wang, X. Molecule confined isolated metal sites enable the electrocatalytic synthesis of hydrogen peroxide. Adv. Mater. 2021, 2104891.
Xia, C.; Xia, Y.; Zhu, P.; Fan, L.; Wang, H. T. Direct electrosynthesis of pure aqueous H2O2 solutions up to 20% by weight using a solid electrolyte. Science 2019, 366, 226–231.
Yang, S.; Verdaguer-Casadevall, A.; Arnarson, L.; Silvioli, L.; Čolić, V.; Frydendal, R.; Rossmeisl, J.; Chorkendorff, I.; Stephens, I. E. L. Toward the decentralized electrochemical production of H2O2: A focus on the catalysis. ACS Catal. 2018, 8, 4064–4081.
Choi, C. H.; Kwon, H. C.; Yook, S.; Shin, H.; Kim, H.; Choi, M. Hydrogen peroxide synthesis via enhanced two-electron oxygen reduction pathway on carbon-coated Pt surface. J. Phys. Chem. C 2014, 118, 30063–30070.
Jirkovský, J. S.; Halasa, M.; Schiffrin, D. J. Kinetics of electrocatalytic reduction of oxygen and hydrogen peroxide on dispersed gold nanoparticles. Phys. Chem. Chem. Phys. 2010, 12, 8042–8053.
Zheng, Z. K.; Ng, Y. H.; Wang, D. W.; Amal, R. Epitaxial growth of Au-Pt-Ni nanorods for direct high selectivity H2O2 production. Adv. Mater. 2016, 28, 9949–9955.
Jirkovský, J. S.; Panas, I.; Ahlberg, E.; Halasa, M.; Romani, S.; Schiffrin, D. J. Single atom hot-spots at Au-Pd nanoalloys for electrocatalytic H2O2 production. J. Am. Chem. Soc. 2011, 133, 19432–19441.
Siahrostami, S.; Verdaguer-Casadevall, A.; Karamad, M.; Deiana, D.; Malacrida, P.; Wickman, B.; Escudero-Escribano, M.; Paoli, E. A.; Frydendal, R.; Hansen, T. W. et al. Enabling direct H2O2 production through rational electrocatalyst design. Nat. Mater. 2013, 12, 1137–1143.
Kim, H. W.; Ross, M. B.; Kornienko, N.; Zhang, L.; Guo, J. H.; Yang, P. D.; McCloskey, B. D. Efficient hydrogen peroxide generation using reduced graphene oxide-based oxygen reduction electrocatalysts. Nat. Catal. 2018, 1, 282–290.
Chen, S. C.; Chen, Z. H.; Siahrostami, S.; Higgins, D.; Nordlund, D.; Sokaras, D.; Kim, T. R.; Liu, Y. Z.; Yan, X. Z.; Nilsson, E. et al. Designing boron nitride islands in carbon materials for efficient electrochemical synthesis of hydrogen peroxide. J. Am. Chem. Soc. 2018, 140, 7851–7859.
Chang, Q. W.; Zhang, P.; Mostaghimi, A. H. B.; Zhao, X. R.; Denny, S. R.; Lee, J. H.; Gao, H. P.; Zhang, Y.; Xin, H. L.; Siahrostami, S. et al. Promoting H2O2 production via 2-electron oxygen reduction by coordinating partially oxidized Pd with defect carbon. Nat. Commun. 2020, 11, 2178.
Lu, Y. Z.; Chen, W. Sub-nanometre sized metal clusters: From synthetic challenges to the unique property discoveries. Chem. Soc. Rev. 2012, 41, 3594–3623.
Liu, W.; Han, L. L.; Wang, H. T.; Zhao, X. R.; Boscoboinik, J. A.; Liu, X. J.; Pao, C. W.; Sun, J. Q.; Zhuo, L. C.; Luo, J. et al. FeMo sub-nanoclusters/single atoms for neutral ammonia electrosynthesis. Nano Energy 2020, 77, 105078.
Wang, Y.; Zheng, X. B.; Wang, D. S. Design concept for electrocatalysts. Nano Res. 2022, 15, 1730–1752.
Jing, H. Y.; Zhu, P.; Zheng, X. B.; Zhang, Z. D.; Wang, D. S.; Li, Y. D. Theory-oriented screening and discovery of advanced energy transformation materials in electrocatalysis. Adv. Powder Mater. in press, https://doi.org/10.1016/j.apmate.2021.10.004.
Wang, Y. C.; Chu, F. L.; Zeng, J.; Wang, Q. J.; Naren, T.; Li, Y. Y.; Cheng, Y.; Lei, Y. P.; Wu, F. X. Single atom catalysts for fuel cells and rechargeable batteries: Principles, advances, and opportunities. ACS Nano 2021, 15, 210–239.
Peng, H. C.; Ren, J.; Wang, Y. C.; Xiong, Y.; Wang, Q. C.; Li, Q.; Zhao, X.; Zhan, L. S.; Zheng, L. R.; Tang, Y. G. et al. One-stone, two birds: Alloying effect and surface defects induced by Pt on Cu2−xSe nanowires to boost C—C bond cleavage for electrocatalytic ethanol oxidation. Nano Energy 2021, 88, 106307.
Qi, D. F.; Liu, S.; Chen, H. H.; Lai, S. H.; Qin, Y. J.; Qiu, Y.; Dai, S.; Zhang, S. S.; Luo, J.; Liu, X. J. Rh nanoparticle functionalized heteroatom-doped hollow carbon spheres for efficient electrocatalytic hydrogen evolution. Mater. Chem. Front. 2021, 5, 3125–3131.
Chen, Y.; Guo, R. J.; Peng, X. Y.; Wang, X. Q.; Liu, X. J.; Ren, J. Q.; He, J.; Zhuo, L. C.; Sun, J. Q.; Liu, Y. F. et al. Highly productive electrosynthesis of ammonia by admolecule-targeting single Ag sites. ACS Nano 2020, 14, 6938–6946.
Han, L. L.; Hou, M. C.; Ou, P. F.; Cheng, H.; Ren, Z. H.; Liang, Z. X.; Boscoboinik, J. A.; Hunt, A.; Waluyo, I.; Zhang, S. S. et al. Local modulation of single-atomic Mn sites for enhanced ambient ammonia electrosynthesis. ACS Catal. 2021, 11, 509–516.
Xie, Z. Y.; Qiu, Y.; Gao, S. S.; Sun, J. Q.; Cao, H. Q.; Zhang, S. S.; Luo, J.; Liu, X. J. Surface oxidized Ag nanofilms towards highly effective CO2 reduction. ChemElectroChem 2021, 8, 3579–3583.
Xu, J.; Lai, S. H.; Qi, D. F.; Hu, M.; Peng, X. Y.; Liu, Y. F.; Liu, W.; Hu, G. Z.; Xu, H.; Li, F. et al. Atomic Fe-Zn dual-metal sites for high-efficiency pH-universal oxygen reduction catalysis. Nano Res. 2021, 14, 1374–1381.
Xia, Y.; Zhao, X. H.; Xia, C.; Wu, Z. Y.; Zhu, P.; Kim, J. Y.; Bai, X. W.; Gao, G. H.; Hu, Y. F.; Zhong, J. et al. Highly active and selective oxygen reduction to H2O2 on boron-doped carbon for high production rates. Nat. Commun. 2021, 12, 4225.
Verdaguer-Casadevall, A.; Deiana, D.; Karamad, M.; Siahrostami, S.; Malacrida, P.; Hansen, T. W.; Rossmeisl, J.; Chorkendorff, I.; Stephens, I. E. L. Trends in the electrochemical synthesis of H2O2: Enhancing activity and selectivity by electrocatalytic site engineering. Nano Lett. 2014, 14, 1603–1608.
Han, G. K.; Zhang, X.; Liu, W.; Zhang, Q. H.; Wang, Z. Q.; Cheng, J.; Yao, T.; Gu, L.; Du, C. Y.; Gao, Y. Z. et al. Substrate strain tunes operando geometric distortion and oxygen reduction activity of CuN2C2 single-atom sites. Nat. Commun. 2021, 12, 6335.
Ding, T.; Liu, X. K.; Tao, Z. N.; Liu, T. Y.; Chen, T.; Zhang, W.; Shen, X. Y.; Liu, D.; Wang, S. C.; Pang, B. B. et al. Atomically precise dinuclear site active toward electrocatalytic CO2 reduction. J. Am. Chem. Soc. 2021, 143, 11317–11324.
Ferrero, G. A.; Preuss, K.; Marinovic, A.; Jorge, A. B.; Mansor, N.; Brett, D. J. L.; Fuertes, A. B.; Sevilla, M.; Titirici, M. M. Fe-N-doped carbon capsules with outstanding electrochemical performance and stability for the oxygen reduction reaction in both acid and alkaline conditions. ACS Nano 2016, 10, 5922–5932.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (Nos. 22075211, 21601136, 51971157, 62005173, and 51621003), Guangdong Province Higher Vocational Colleges & Schools Pearl River Scholar Funded Scheme (2016), Guangdong Third Generation Semiconductor Engineering Technology Development Center (No. 2020GCZX007), Science, Technology, and Innovation Commission of Shenzhen Municipality (No. RCBS20200714114818140), China Postdoctoral Science Foundation (No. 2019M663118), and School level scientific research project of Shenzhen Institute of information technology (No. PT2019E002).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Jin, M., Liu, W., Sun, J. et al. Highly dispersed Ag clusters for active and stable hydrogen peroxide production. Nano Res. 15, 5842–5847 (2022). https://doi.org/10.1007/s12274-022-4208-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-4208-7