Abstract
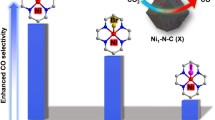
Modulating the local coordination structure of metal single-atom catalysts (SACs) is extensively employed to tune the catalytic activity, but rarely involved in regulating the reaction pathway which fundamentally determines the product selectivity. Herein, we report that the product selectivity of electrochemical CO2 reduction (CO2RR) on the single-atom indium-NxC4−x (1 ≤ x ≤ 4) catalysts could be tuned from formate to CO by varying the carbon and nitrogen occupations in the first coordination sphere. Surprisingly, the optimal In SAC showed great promise for CO production with the maximum Faradic efficiency of 97%, greatly different from the reported In-based catalysts where the formate is the dominant product. Combined experimental verifications and theoretical simulations reveal that the selectivity switch from formate to CO on In SACs originates from active sites shift from indium center to the indium-adjacent carbon atom, where the indium site favors formate formation and the indium-adjacent carbon site prefers the CO pathway. The present work suggests the active sites in metal SACs may shift from the widely accepted metal center to surrounding carbon atoms, thereby offering a new implication to revisit the active sites for metal SACs.

Similar content being viewed by others
References
Franco, F.; Rettenmaier, C.; Jeon, H. S.; Roldan Cuenya, B. Transition metal-based catalysts for the electrochemical CO2 reduction: From atoms and molecules to nanostructured materials. Chem. Soc. Rev. 2020, 49, 6884–6946.
De Luna, P.; Hahn, C.; Higgins, D.; Jaffer, S. A.; Jaramillo, T. F.; Sargent, E. H. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science 2019, 364, eaav3506.
Shih, C. F.; Zhang, T.; Li, J. H.; Bai, C. L. Powering the Future with Liquid Sunshine. Joule 2018, 2, 1925–1949.
Yang, H. P.; Wu, Y.; Lin, Q.; Fan, L. D.; Chai, X. Y.; Zhang, Q. L.; Liu, J. H.; He, C. X.; Lin, Z. Q. Composition tailoring via N and S co-doping and structure tuning by constructing hierarchical pores: Metal-free catalysts for high-performance electrochemical reduction of CO2. Angew. Chem., Int. Ed. 2018, 57, 15476–15480.
Zhang, M.; Xuan, X. X.; Wang, W. L.; Ma, C. Y.; Lin, Z. Q. Anode photovoltage compensation-enabled synergistic CO2 photoelectrocatalytic reduction on a flower-like graphene-decorated Cu foam cathode. Adv. Funct. Mater. 2020, 30, 2005983.
Nitopi, S.; Bertheussen, E.; Scott, S. B.; Liu, X. Y.; Engstfeld, A. K.; Horch, S.; Seger, B.; Stephens, I. E. L.; Chan, K.; Hahn, C. et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 2019, 119, 7610–7672.
Masel, R. I.; Liu, Z. C.; Yang, H. Z.; Kaczur, J. J.; Carrillo, D.; Ren, S. X.; Salvatore, D.; Berlinguette, C. P. An industrial perspective on catalysts for low-temperature CO2 electrolysis. Nat. Nanotechnol. 2021, 16, 118–128.
Zhang, Y.; Guo, S. X.; Zhang, X. L.; Bond, A. M.; Zhang, J. Mechanistic understanding of the electrocatalytic CO2 reduction reaction-New developments based on advanced instrumental techniques. Nano Today 2020, 31, 100835.
Yu, D.; Gao, L.; Sun, T. L.; Guo, J. C.; Yuan, Y. L.; Zhang, J. W.; Li, M. F.; Li, X. X.; Liu, M. C.; Ma, C. et al. Strain-stabilized metastable face-centered tetragonal gold overlayer for efficient CO2 electroreduction. Nano Lett. 2021, 21, 1003–1010.
Yoshio, H.; Katsuhei, K.; Akira, M.; Shin, S. Production of methane and ethylene in electrochemical reduction of carbon dioxide at copper electrode in aqueous hydrogencarbonate solution. Chem. Lett. 1986, 15, 897–898.
Yoshio, H.; Katsuhei, K.; Shin, S. Production of CO and CH4 in electrochemical reduction of CO2 at metal electrodes in aqueous hydrogencarbonate solution. Chem. Lett. 1985, 14, 1695–1698.
Asadi, M.; Kim, K.; Liu, C.; Addepalli, A. V.; Abbasi, P.; Yasaei, P.; Phillips, P.; Behranginia, A.; Cerrato, J. M.; Haasch, R. et al. Nanostructured transition metal dichalcogenide electrocatalysts for CO2 reduction in ionic liquid. Science 2016, 353, 467–470.
Chen, J. Y.; Wang, T. T.; Li, Z. J.; Yang, B.; Zhang, Q. H.; Lei, L. C.; Feng, P. Y.; Hou, Y. Recent progress and perspective of electrochemical CO2 reduction towards C2–C5 products over non-precious metal heterogeneous electrocatalysts. Nano Res. 2021, 14, 3188–3207.
Gao, D. F.; Zhou, H.; Wang, J.; Miao, S.; Yang, F.; Wang, G. X.; Wang, J. G.; Bao, X. H. Size-dependent electrocatalytic reduction of CO2 over Pd nanoparticles. J. Am. Chem. Soc. 2015, 137, 4288–4291.
Zhu, W. L.; Zhang, Y. J.; Zhang, H. Y.; Lv, H. F.; Li, Q.; Michalsky, R.; Peterson, A. A.; Sun, S. H. Active and selective conversion of CO2 to CO on ultrathin Au nanowires. J. Am. Chem. Soc. 2014, 136, 16132–16135.
Back, S.; Lim, J.; Kim, N. Y.; Kim, Y. H.; Jung, Y. Single-atom catalysts for CO2 electroreduction with significant activity and selectivity improvements. Chem. Sci. 2017, 8, 1090–1096.
Varela, A. S.; Ju, W.; Bagger, A.; Franco, P.; Rossmeisl, J.; Strasser, P. Electrochemical reduction of CO2 on metal-nitrogen-doped carbon catalysts. ACS Catal. 2019, 9, 7270–7284.
Zhang, Z.; Ma, C.; Tu, Y. C.; Si, R.; Wei, J.; Zhang, S. H.; Wang, Z.; Li, J. F.; Wang, Y.; Deng, D. H. Multiscale carbon foam confining single iron atoms for efficient electrocatalytic CO2 reduction to CO. Nano Res. 2019, 12, 2313–2317.
Zhang, J. C.; Cai, W. Z.; Hu, F. X.; Yang, H. B.; Liu, B. Recent advances in single atom catalysts for the electrochemical carbon dioxide reduction reaction. Chem. Sci. 2021, 12, 6800–6819.
Su, X.; Yang, X. F.; Huang, Y. Q.; Liu, B.; Zhang, T. Single-atom catalysis toward efficient CO2 conversion to CO and formate products. Acc. Chem. Res. 2019, 52, 656–664.
Chen, S. H.; Li, W. H.; Jiang, W. J.; Yang, J. R.; Zhu, J. X.; Wang, L. Q.; Ou, H. H.; Zhuang, Z. C.; Chen, M. Z.; Sun, X. H. et al. MOF encapsulating N-heterocyclic carbene-ligated copper single-atom site catalyst towards efficient methane electrosynthesis. Angew. Chem., Int. Ed. 2022, 61, e202114450.
Sun, X. H.; Tuo, Y. X.; Ye, C. L.; Chen, C.; Lu, Q.; Li, G. N.; Jiang, P.; Chen, S. H.; Zhu, P.; Ma, M. et al. Phosphorus induced electron localization of single iron sites for boosted CO2 electroreduction reaction. Angew. Chem., Int. Ed. 2021, 60, 23614–23618.
Jiang, K.; Siahrostami, S.; Zheng, T. T.; Hu, Y. F.; Hwang, S.; Stavitski, E.; Peng, Y. D.; Dynes, J.; Gangisetty, M.; Su, D. et al. Isolated Ni single atoms in graphene nanosheets for high-performance CO2 reduction. Energy Environ. Sci. 2018, 11, 893–903.
Jiang, K.; Siahrostami, S.; Akey, A. J.; Li, Y. B.; Lu, Z. Y.; Lattimer, J.; Hu, Y. F.; Stokes, C.; Gangishetty, M.; Chen, G. X. et al. Transition-metal single atoms in a graphene shell as active centers for highly efficient artificial photosynthesis. Chem. 2017, 3, 950–960.
Zhang, E. H.; Wang, T.; Yu, K.; Liu, J.; Chen, W. X; Li, A.; Rong, H. P.; Lin, R.; Ji, S. F.; Zheng, X. S. et al. Bismuth single atoms resulting from transformation of metal-organic frameworks and their use as electrocatalysts for CO2 reduction. J. Am. Chem. Soc. 2019, 141, 16569–16573.
Cui, X.; Gao, L. K.; Lei, S.; Liang, S.; Zhang, J. W.; Sewell, C. D.; Xue, W. D.; Liu, Q.; Lin, Z. Q.; Yang, Y. K. Simultaneously crafting single-atomic fe sites and graphitic layer-wrapped Fe3C nanoparticles encapsulated within mesoporous carbon tubes for oxygen reduction. Adv. Funct. Mater. 2021, 31, 2009197.
Qu, Q. Y.; Ji, S. F.; Chen, Y. J.; Wang, D. S.; Li, Y. D. The atomic-level regulation of single-atom site catalysts for the electrochemical CO2 reduction reaction. Chem. Sci. 2021, 12, 4201–4215.
Li, X. Y.; Rong, H. P.; Zhang, J. T.; Wang, D. S.; Li, Y. D. Modulating the local coordination environment of single-atom catalysts for enhanced catalytic performance. Nano Res. 2020, 13, 1842–1855.
Hu, M. Y.; Li, S. N.; Zheng, S. S.; Liang, X. H.; Zheng, J. X.; Pan, F. Tuning single-atom catalysts of nitrogen-coordinated transition metals for optimizing oxygen evolution and reduction reactions. J. Phys. Chem. C 2020, 124, 13168–13176.
Shang, H. S.; Zhou, X. Y.; Dong, J. C.; Li, A.; Zhao, X.; Liu, Q. H.; Lin, Y.; Pei, J. J.; Li, Z.; Jiang, Z. L. et al. Engineering unsymmetrically coordinated Cu-S1N3 single atom sites with enhanced oxygen reduction activity. Nat. Commun. 2020, 11, 3049.
Pan, Y.; Chen, Y. J.; Wu, K. L.; Chen, Z.; Liu, S. J.; Cao, X.; Cheong, W. C.; Meng, T.; Luo, J.; Zheng, L. R. et al. Regulating the coordination structure of single-atom Fe-NxCy catalytic sites for benzene oxidation. Nat. Commun. 2019, 10, 4290.
Zhang, J. T.; Zhang, M.; Zeng, Y.; Chen, J. S.; Qiu, L. X.; Zhou, H.; Sun, C. J.; Yu, Y.; Zhu, C. Z.; Zhu, Z. H. Single Fe atom on hierarchically porous S, N-codoped nanocarbon derived from porphyra enable boosted oxygen catalysis for rechargeable Zn-Air batteries. Small 2019, 15, 1900307.
Wang, X. Q.; Chen, Z.; Zhao, X. Y.; Yao, T.; Chen, W. X.; You, R.; Zhao, C. M.; Wu, G.; Wang, J.; Huang, W. X. et al. Regulation of coordination number over single co sites: Triggering the efficient electroreduction of CO2. Angew. Chem., Int. Ed. 2018, 57, 1944–1948.
Rong, X.; Wang, H. J.; Lu, X. L.; Si, R.; Lu, T. B. Controlled synthesis of a vacancy-defect single-atom catalyst for boosting CO2 electroreduction. Angew. Chem., Int. Ed. 2020, 59, 1961–1965.
Jia, M. W.; Hong, S.; Wu, T. S.; Li, X.; Soo, Y. L.; Sun, Z. Y. Single Sb sites for efficient electrochemical CO2 reduction. Chem. Commun. 2019, 55, 12024–12027.
Jiang, Z. L.; Wang, T.; Pei, J. J.; Shang, H. S.; Zhou, D. N.; Li, H. J.; Dong, J. C.; Wang, Y.; Cao, R.; Zhuang, Z. B. et al. Discovery of main group single Sb-N4 active sites for CO2 electroreduction to formate with high efficiency. Energy Environ. Sci. 2020, 13, 2856–2863.
Tang, C.; Chen, L.; Li, H. J.; Li, L. Q.; Jiao, Y.; Zheng, Y.; Xu, H. L.; Davey, K.; Qiao, S. Z. Tailoring acidic oxygen reduction selectivity on single-atom catalysts via modification of first and second coordination spheres. J. Am. Chem. Soc. 2021, 143, 7819–7827.
Jing, H. Y.; Zhu, P.; Zheng, X. B.; Zhang, Z. D.; Wang, D. S.; Li, Y. D. Theory-oriented screening and discovery of advanced energy transformation materials in electrocatalysis. Adv. Powder Mater., in press, DOI:https://doi.org/10.1016/j.apmate.2021.10.004.
Wang, Y.; Zheng, X. B.; Wang, D. S. Design concept for electrocatalysts. Nano Res. 2022, 15, 1730–1752.
Jing, H. Y.; Zhao, Z. Y.; Zhang, J. W.; Zhu, C.; Liu, W.; Li, N. N.; Hao, C.; Shi, Y. T.; Wang, D. S. Atomic evolution of metal-organic frameworks into CO-N3 coupling vacancies by cooperative cascade protection strategy for promoting triiodide reduction. J. Phys. Chem. C 2021, 125, 6147–6156.
Jing, H. Y.; Liu, W.; Zhao, Z. Y.; Zhang, J. W.; Zhu, C.; Shi, Y. T.; Wang, D. S.; Li, Y. D. Electronics and coordination engineering of atomic cobalt trapped by oxygen-driven defects for efficient cathode in solar cells. Nano Energy 2021, 89, 106365.
Lu, P. L.; Tan, X.; Zhao, H. T.; Xiang, Q.; Liu, K. L.; Zhao, X. X.; Yin, X. M.; Li, X. Z.; Hai, X.; Xi, S. B. et al. Atomically dispersed indium sites for selective CO2 electroreduction to formic acid. ACS Nano 2021, 15, 5671–5678.
Wang, H.; Jia, J.; Song, P. F.; Wang, Q.; Li, D. B.; Min, S. X.; Qian, C. X.; Wang, L.; Li, Y. F.; Ma, C. et al. Efficient electrocatalytic reduction of CO2 by nitrogen-doped nanoporous carbon/carbon nanotube membranes: A step towards the electrochemical CO2 Refinery. Angew. Chem., Int. Ed. 2017, 56, 7847–7852.
Yang, H. P.; Wu, Y.; Li, G. D.; Lin, Q.; Hu, Q.; Zhang, Q. L.; Liu, J. H.; He, C. X. Scalable production of efficient single-atom copper decorated carbon membranes for CO2 electroreduction to methanol. J. Am. Chem. Soc. 2019, 141, 12717–12723.
Shang, H. S.; Wang, T.; Pei, J. J.; Jiang, Z. L.; Zhou, D. N.; Wang, Y.; Li, H. J.; Dong, J. C.; Zhuang, Z. B.; Chen, W. X. et al. Design of a single-atom indiumδ+-N4 interface for efficient electroreduction of CO2 to formate. Angew. Chem., Int. Ed. 2020, 59, 22465–22469.
Guo, W. W.; Tan, X. X.; Bi, J. H.; Xu, L.; Yang, D. X.; Chen, C. J.; Zhu, Q. G.; Ma, J.; Tayal, A.; Ma, J. Y. et al. Atomic indium catalysts for switching CO2 electroreduction products from formate to CO. J. Am. Chem. Soc. 2021, 143, 6877–6885.
Luo, F.; Roy, A.; Silvioli, L.; Cullen, D. A.; Zitolo, A.; Sougrati, M. T.; Oguz, I. C.; Mineva, T.; Teschner, D.; Wagner, S. et al. P-block single-metal-site tin/nitrogen-doped carbon fuel cell cathode catalyst for oxygen reduction reaction. Nat. Mater. 2020, 19, 1215–1223.
Ren, W. H.; Tan, X.; Yang, W. F.; Jia, C.; Xu, S. M.; Wang, K. X.; Smith, S. C.; Zhao, C. Isolated diatomic Ni-Fe metal-nitrogen sites for synergistic electroreduction of CO2. Angew. Chem., Int. Ed. 2019, 58, 6972–6976.
Lu, Q.; Rosen, J.; Zhou, Y.; Hutchings, G. S.; Kimmel, Y. C.; Chen, J. G.; Jiao, F. A selective and efficient electrocatalyst for carbon dioxide reduction. Nat. Commun. 2014, 5, 3242.
Liu, K.; Smith, W. A.; Burdyny, T. Introductory guide to assembling and operating gas diffusion electrodes for electrochemical CO2 reduction. ACS Energy Lett. 2019, 4, 639–643.
Zheng, T. T.; Jiang, K.; Ta, N.; Hu, Y. F.; Zeng, J.; Liu, J. Y.; Wang, H. T. Large-scale and highly selective CO2 electrocatalytic reduction on nickel single-atom catalyst. Joule 2019, 3, 265–278.
Gabardo, C. M.; O’Brien, C. P.; Edwards, J. P.; McCallum, C.; Xu, Y.; Dinh, C. T.; Li, J.; Sargent, E. H.; Sinton, D. Continuous carbon dioxide electroreduction to concentrated multi-carbon products using a membrane electrode assembly. Joule 2019, 3, 2777–2791.
Yang, L.; Cheng, D. J.; Xu, H. X.; Zeng, X. F.; Wan, X.; Shui, J. L.; Xiang, Z. H.; Cao, D. P. Unveiling the high-activity origin of single-atom iron catalysts for oxygen reduction reaction. Proc. Natl. Acad. Sci. USA 2018, 115, 6626–6631.
Zhang, B. X.; Zhang, J. L.; Shi, J. B.; Tan, D. X.; Liu, L. F.; Zhang, F. Y.; Lu, C.; Su, Z. Z.; Tan, X. N.; Cheng, X. Y. et al. Manganese acting as a high-performance heterogeneous electrocatalyst in carbon dioxide reduction. Nat. Commun. 2019, 10, 2980.
Ni, W. P.; Liu, Z. X.; Zhang, Y.; Ma, C.; Deng, H. Q.; Zhang, S. G.; Wang, S. Y. Electroreduction of carbon dioxide driven by the intrinsic defects in the carbon plane of a single Fe-N4 site. Adv. Mater. 2021, 33, 2003238.
Liu, C.; Li, H.; Liu, F.; Chen, J. S.; Yu, Z. X.; Yuan, Z. W.; Wang, C. J.; Zheng, H. L.; Henkelman, G.; Wei, L. et al. Intrinsic activity of metal centers in metal-nitrogen-carbon single-atom catalysts for hydrogen peroxide synthesis. J. Am. Chem. Soc. 2020, 142, 21861–21871.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 21905089, 2021RC3065, and 2021RC2053), the National Key Research and Development Program of China (No. 2021YFA1502000), the Science and Technology Innovation Program of Hunan Province (Nos. 2021RC3065 and 2021RC2053), Hunan Provincial Natural Science Foundation of China (No. 2020JJ2001), Shenzhen Science and Technology Program (No. JCYJ20210324120800002), and the Hefei National Laboratory for Physical Sciences at the Microscale (No. KF2020108). The calculations were performed on the supercomputing center of the University of Science and Technology of China (USTC-SCC) and Guangzhou-SCC.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2022_4177_MOESM1_ESM.pdf
Tuning the reaction path of CO2 electroreduction reaction on indium single-atom catalyst: Insights into the active sites
Rights and permissions
About this article
Cite this article
Zhang, J., Zeng, G., Chen, L. et al. Tuning the reaction path of CO2 electroreduction reaction on indium single-atom catalyst: Insights into the active sites. Nano Res. 15, 4014–4022 (2022). https://doi.org/10.1007/s12274-022-4177-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-4177-x