Abstract
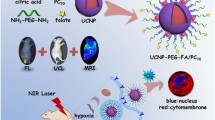
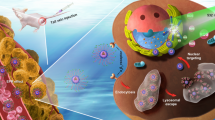
Photodynamic therapy (PDT) is a promising and non-invasive treatment for various cancers. Although nuclear PDT has considerable therapeutic prospects, it is still hindered by the non-specific recognition of tumor tissues or the degradation of nuclear targeting cationic groups by enzymes in the blood. Herein, a hierarchical targeted and controlled release strategy is proposed by using folate-modified poly-β-cyclodextrin (poly-β-CD) as a nano-carrier for loading nuclear localization signals (NLSs)-conjugated photosensitizer PAP (PAP = pyropheophorbide a—PAAKRVKLD). Excitingly, the obtained FA-CD@PAP (FA = folic acid) and nanoparticles (NPs) can specifically recognize tumor cells overexpressing folate receptors (FR) to remarkedly enhance the intracellular accumulation. Furthermore, the encapsulated PAP can be released under acidic conditions to realize precise nuclear localization. The reactive oxygen species (ROS) generated by the intranuclear-accumulated PAP upon irradiation can oxidize and destroy DNA chains or DNA repair enzymes instantaneously, which can directly induce cell death. As a result, FA-CD@PAP NPs exhibit excellent tumor regression and negligible side effects. This work provides an intelligent nuclear-targeted delivery strategy for in situ nuclear PDT with extremely prominent efficacy and high biological safety.

Similar content being viewed by others
References
Kuimova, M. K.; Botchway, S. W.; Parker, A. W.; Balaz, M.; Collins, H. A.; Anderson, H. L.; Suhling, K.; Ogilby, P. R. Imaging intracellular viscosity of a single cell during photoinduced cell death. Nat. Chem. 2009, 1, 69–73.
Lovell, J. F.; Liu, T. W. B.; Chen, J.; Zheng, G. Activatable photosensitizers for imaging and therapy. Chem. Rev. 2010, 110, 2839–2857.
Li, F.; Chen, C.; Yang, X. X.; He, X. Y.; Zhao, Z. Y.; Li, J.; Yu, Y.; Yang, X. Z.; Wang, J. Acetal-linked hyperbranched polyphosphoester nanocarriers loaded with chlorin e6 for pH-activatable photodynamic therapy. ACS Appl. Mater. Interfaces 2018, 10, 21198–21205.
Zhou, Z. J.; Song, J. B.; Nie, L. M.; Chen, X. Y. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 2016, 45, 6597–6626.
Ha, S. Y. Y.; Zhou, Y. M.; Fong, W. P.; Ng, D. K. P. Multifunctional molecular therapeutic agent for targeted and controlled dual chemo- and photodynamic therapy. J. Med. Chem. 2020, 63, 8512–8523.
Zhang, F. L.; Song, M. R.; Yuan, G. K.; Ye, H. N.; Tian, Y.; Huang, M. D.; Xue, J. P.; Zhang, Z. H.; Liu, J. Y. A molecular combination of Zinc(II) phthalocyanine and tamoxifen derivative for dual targeting photodynamic therapy and hormone therapy. J. Med. Chem. 2017, 60, 6693–6703.
Celli, J. P.; Spring, B. Q.; Rizvi, I.; Evans, C. L.; Samkoe, K. S.; Verma, S.; Pogue, B. W.; Hasan, T. Imaging and photodynamic therapy: Mechanisms, monitoring, and optimization. Chem. Rev. 2010, 110, 2795–2838.
Moore, C. M.; Pendse, D.; Emberton, M. Photodynamic therapy for prostate cancer-a review of current status and future promise. Nat. Clin. Pract. Urol. 2009, 6, 18–30.
Zhang, C.; Qin, W. J.; Bai, X. F.; Zhang, X. Z. Nanomaterials to relieve tumor hypoxia for enhanced photodynamic therapy. Nano Today 2020, 35, 100960.
Lismont, M.; Dreesen, L.; Wuttke, S. Metal-organic framework nanoparticles in photodynamic therapy: Current status and perspectives. Adv. Funct. Mater. 2017, 27, 1606314.
Guo, R. W.; Yang, G.; Feng, Z. J.; Zhu, Y. J.; Yang, P. X.; Song, H. J.; Wang, W. W.; Huang, P. S.; Zhang, J. H. Glutathione-induced amino-activatable micellar photosensitization platform for synergistic redox modulation and photodynamic therapy. Biomater. Sci. 2018, 6, 1238–1249.
Feng, C.; Zhu, D. L.; Chen, L.; Lu, Y. L.; Liu, J.; Kim, N. Y.; Liang, S. J.; Zhang, X.; Lin, Y.; Ma, Y. B. et al. Targeted delivery of chlorin e6 via redox sensitive diselenide-containing micelles for improved photodynamic therapy in cluster of differentiation 44-overexpressing breast cancer. Front. Pharmacol. 2019, 10, 369.
Yan, S.; Huang, Q.; Chen, J.; Song, X.; Chen, Z.; Huang, M.; Xu, P.; Zhang, J. Tumor-targeting photodynamic therapy based on folate-modified polydopamine nanoparticles. Int. J. Nanomed. 2019, 14, 6799–6812.
Huang, Z.; Xu, H. P.; Meyers, A. D.; Musani, A. I.; Wang, L. W.; Tagg, R.; Barqawi, A. B.; Chen, Y. K. Photodynamic therapy for treatment of solid tumors-potential and technical challenges. Technol. Cancer Res. Treat. 2008, 7, 309–320.
Sharman, W. M.; Allen, C. M.; van Lier, J. E. Photodynamic therapeutics: Basic principles and clinical applications. Drug Discov. Today 1999, 4, 507–517.
Ethirajan, M.; Chen, Y. H.; Joshi, P.; Pandey, R. K. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 2011, 40, 340–362.
Liu, C. H.; Cao, Y.; Cheng, Y. R.; Wang, D. D.; Xu, T. L.; Su, L.; Zhang, X. J.; Dong, H. F. An open source and reduce expenditure ROS generation strategy for chemodynamic/photodynamic synergistic therapy. Nat. Commun. 2020, 11, 1735.
Yu, Z. Z.; Sun, Q. Q.; Pan, W.; Li, N.; Tang, B. A near-infrared triggered nanophotosensitizer inducing domino effect on mitochondrial reactive oxygen species burst for cancer therapy. ACS Nano 2015, 9, 11064–11074.
Moor, A. C. E. Signaling pathways in cell death and survival after photodynamic therapy. J. Photochem. Photobiol. B: Biol. 2000, 57, 1–13.
Ngen, E.; Rajaputra, P.; You, Y. Evaluation of delocalized lipophilic cationic dyes as delivery vehicles for photosensitizers to mitochondria. Bioorg. Bioorgan. Med. Chem. 2009, 17, 6631–6640.
Hatz, S.; Lambert, J. D. C.; Ogilby, P. R. Measuring the lifetime of singlet oxygen in a single cell: Addressing the issue of cell viability. Photochem. Photobiol. Sci. 2007, 6, 1106–1116.
Zhang, Y. Y.; Wang, L. K.; Rao, Q. P.; Bu, Y. C.; Xu, T. R.; Zhu, X. J.; Zhang, J.; Tian, Y. P.; Zhou, H. P. Tuning the hydrophobicity of pyridinium-based probes to realize the mitochondria-targeted photodynamic therapy and mitophagy tracking. Sens. Actuators B: Chem. 2020, 321, 128460.
Chen, X. H.; Li, Y. X.; Li, S. W.; Gao, M.; Ren, L.; Tang, B. Z. Mitochondria- and lysosomes-targeted synergistic chemophotodynamic therapy associated with self-monitoring by dual light-up fluorescence. Adv. Funct. Mater. 2018, 28, 1804362.
Xu, J. S.; Zeng, F.; Wu, H.; Hu, C. P.; Wu, S. Z. Enhanced photodynamic efficiency achieved via a dual-targeted strategy based on photosensitizer/micelle structure. Biomacromolecules 2014, 15, 4249–4259.
Song, X. Y.; Yue, Z. H.; Hong, T. T.; Wang, Z. H.; Zhang, S. S. Sandwich-structured upconversion nanoprobes coated with a thin silica layer for mitochondria-targeted cooperative photodynamic therapy for solid malignant tumors. Anal. Chem. 2019, 91, 8549–8557.
Pereira, P. M. R.; Silva, S.; Bispo, M.; Zuzarte, M.; Gomes, C.; Girão, H.; Cavaleiro, J. A. S.; Ribeiro, C. A. F.; Tomé, J. P. C.; Fernandes, R. Mitochondria-targeted photodynamic therapy with a galactodendritic chlorin to enhance cell death in resistant bladder cancer cells. Bioconjug. Chem. 2016, 27, 2762–2769.
Xiao, Q. C.; Lin, H. R.; Wu, J.; Pang, X.; Zhou, Q. M.; Jiang, Y.; Wang, P.; Leung, W. N.; Lee, H.; Jiang, S. et al. Pyridine-embedded phenothiazinium dyes as lysosome-targeted photosensitizers for highly efficient photodynamic antitumor therapy. J. Med. Chem. 2020, 63, 4896–4907.
Khaddaj, R.; Mari, M.; Cottier, S.; Reggiori, F.; Schneiter, R. The surface of lipid droplets constitutes a barrier for endoplasmic reticulum-resident integral membrane proteins. J. Cell Sci. 2022, 135, jcs256206.
Li, H.; Liu, C.; Zeng, Y. P.; Hao, Y. H.; Huang, J. W.; Yang, Z. Y.; Li, R. Nanoceria-mediated drug delivery for targeted photodynamic therapy on drug-resistant breast cancer. ACS Appl. Mater. Interfaces 2016, 8, 31510–31523.
Rosado, A.; Bayer, E. M. Geometry and cellular function of organelle membrane interfaces. Plant Physiol. 2021, 185, 650–662.
Yin, J. L.; Huang, L.; Wu, L. L.; Li, J. F.; James, T. D.; Lin, W. Y. Small molecule based fluorescent chemosensors for imaging the microenvironment within specific cellular regions. Chem. Soc. Rev. 2021, 50, 12098–12150.
Saito, A.; Imaizumi, K. Unfolded protein response-dependent communication and contact among endoplasmic reticulum, mitochondria, and plasma membrane. Int. J. Mol. Sci. 2018, 19, 3215.
Tilokani, L.; Nagashima, S.; Paupe, V.; Prudent, J. Mitochondrial dynamics: Overview of molecular mechanisms. Essays Biochem. 2018, 62, 341–360.
Yue, J.; Liang, L. J.; Shen, Y. T.; Guan, X.; Zhang, J.; Li, Z. Y.; Deng, R.; Xu, S. P.; Liang, C. Y.; Shi, W. et al. Investigating dynamic molecular events in melanoma cell nucleus during photodynamic therapy by SERS. Front. Chem. 2019, 6, 665.
Huo, S. D.; Jin, S. B.; Ma, X. W.; Xue, X. D.; Yang, K. N.; Kumar, A.; Wang, P. C.; Zhang, J. C.; Hu, Z. B.; Liang, X. J. Ultrasmall gold nanoparticles as carriers for nucleus-based gene therapy due to size-dependent nuclear entry. ACS Nano 2014, 8, 5852–5862.
Lim, S. H.; Thivierge, C.; Nowak-Sliwinska, P.; Han, J. Y.; van den Bergh, H.; Wagnières, G.; Burgess, K.; Lee, H. B. In vitro and in vivo photocytotoxicity of boron dipyrromethene derivatives for photodynamic therapy. J. Med. Chem. 2010, 53, 2865–2874.
Derycke, A. S. L.; De Witte, P. A. M. Liposomes for photodynamic therapy. Adv. Drug Deliv. Rev. 2004, 56, 17–30.
Cheng, Y.; Samia, A. C.; Meyers, J. D.; Panagopoulos, I.; Fei, B. W.; Burda, C. Highly efficient drug delivery with gold nanoparticle vectors for in vivo photodynamic therapy of cancer. J. Am. Chem. Soc. 2008, 130, 10643–10647.
Tian, B.; Wang, C.; Zhang, S.; Feng, L. Z.; Liu, Z. Photothermally enhanced photodynamic therapy delivered by nano-graphene oxide. ACS Nano 2011, 5, 7000–7009.
Khan, M.; Kumar, B.; Zhao, Y.; Hassan, M.; Liu, Y.; Wang, L.; Liu, H.; Jiang, W. Stepwise-activatable hypoxia triggered nanocarrier-based photodynamic therapy for effective synergistic bioreductive chemotherapy. Biomaterials 2020, 245, 119982.
Marfori, M.; Mynott, A.; Ellis, J. J.; Mehdi, A. M.; Saunders, N. F. W.; Curmi, P. M.; Forwood, J. K.; Bodén, M.; Kobe, B. Molecular basis for specificity of nuclear import and prediction of nuclear localization. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2011, 1813, 1562–1577.
Dosio, F.; Arpicco, S.; Stella, B.; Fattal, E. Hyaluronic acid for anticancer drug and nucleic acid delivery. Adv. Drug Deliv. Rev. 2016, 97, 204–236.
Belting, M.; Sandgren, S.; Wittrup, A. Nuclear delivery of macromolecules: Barriers and carriers. Adv. Drug Deliv. Rev. 2005, 57, 505–527.
Chow, K. H.; Factor, R. E.; Ullman, K. S. The nuclear envelope environment and its cancer connections. Nat. Rev. Cancer 2012, 12, 196–209.
Zhang, Y. Y.; Lv, F.; Cheng, Y. R.; Yuan, Z. P.; Yang, F.; Liu, C. H.; Cao, Y.; Zhang, K.; Lu, H. T.; Zada, S. et al. Pd@Au bimetallic nanoplates decorated mesoporous MnO2 for synergistic nucleus-targeted NIR-II photothermal and hypoxia-relieved photodynamic therapy. Adv. Healthc. Mater. 2020, 9, 1901528.
Han, K.; Zhang, W. Y.; Zhang, J.; Lei, Q.; Wang, S. B.; Liu, J. W.; Zhang, X. Z.; Han, H. Y. Acidity-triggered tumor-targeted chimeric peptide for enhanced intra-nuclear photodynamic therapy. Adv. Funct. Mater. 2016, 26, 4351–4361.
Pan, L. M.; Liu, J. N.; Shi, J. L. Intranuclear photosensitizer delivery and photosensitization for enhanced photodynamic therapy with ultralow irradiance. Adv. Funct. Mater. 2014, 24, 7318–7327.
Wan, G. Y.; Cheng, Y. Y.; Song, J.; Chen, Q.; Chen, B. W.; Liu, Y. Y.; Ji, S. L.; Chen, H. L.; Wang, Y. S. Nucleus-targeting near-infrared nanoparticles based on TAT peptide-conjugated IR780 for photo-chemotherapy of breast cancer. Chem. Eng. J. 2020, 380, 122458.
Makkerh, J. P. S.; Dingwall, C.; Laskey, R. A. Comparative mutagenesis of nuclear localization signals reveals the importance of neutral and acidic amino acids. Curr. Biol. 1996, 6, 1025–1027.
Dang, C. V.; Lee, W. M. Identification of the human c-myc protein nuclear translocation signal. Mol. Cell Biol. 1988, 8, 4048–4054.
Day, A. H.; Übler, M. H.; Best, H. L.; Lloyd-Evans, E.; Mart, R. J.; Fallis, I. A.; Allemann, R. K.; Al-Wattar, E. A. H.; Keymer, N. I.; Buurma, N. J.; Pope, S. J. A. Targeted cell imaging properties of a deep red luminescent iridium(III) complex conjugated with a c-Myc signal peptide. Chem. Sci. 2020, 11, 1599–1606.
Zhang, Y. M.; Liu, Y. H.; Liu, Y. Cyclodextrin-based multistimuli-responsive supramolecular assemblies and their biological functions. Adv. Mater. 2020, 32, 1806158.
Dai, X. Y.; Dong, X. Y.; Liu, Z. X.; Liu, G. X.; Liu, Y. Controllable singlet oxygen generation in water based on cyclodextrin secondary assembly for targeted photodynamic therapy. Biomacromolecules 2020, 21, 5369–5379.
Hu, Q. D.; Tang, G. P.; Chu, P. K. Cyclodextrin-based host-guest supramolecular nanoparticles for delivery: From design to applications. Acc. Chem. Res. 2014, 47, 2017–2025.
He, L. L.; Yang, X. H.; Zhao, F.; Wang, K. M.; Wang, Q.; Liu, J. B.; Huang, J.; Li, W. S.; Yang, M. Self-assembled supramolecular nanoprobes for ratiometric fluorescence measurement of intracellular pH values. Anal. Chem. 2015, 87, 2459–2465.
Li, B. J.; Feng, Z. Z.; He, L. L.; Li, W. S.; Wang, Q.; Liu, J. B.; Huang, J.; Zheng, Y.; Ma, Y. Y.; Yang, X. H. et al. Self-assembled supramolecular nanoparticles for targeted delivery and combination chemotherapy. ChemMedChem 2018, 13, 2037–2044.
Koopmans, C.; Ritter, H. Formation of Physical Hydrogels via Host-Guest Interactions of β-Cyclodextrin Polymers and Copolymers Bearing Adamantyl Groups. Macromolecules 2008, 41, 7418–7422.
Huang, L.; Tonelli, A. E. Polymer inclusion compounds. J. Macromol. Sci. C 1998, 38, 781–837.
Fan, W. B.; Xu, Y. D.; Li, Z.; Li, Q. Folic acid-modified β-cyclodextrin nanoparticles as drug delivery to load DOX for liver cancer therapeutics. Soft Mater. 2019, 17, 437–447.
Ang, C. Y.; Tan, S. Y.; Teh, C.; Lee, J. M.; Wong, M. F. E.; Qu, Q. Y.; Poh, L. Q.; Li, M. H.; Zhang, Y. Y.; Korzh, V. et al. Redox and pH dual responsive polymer based nanoparticles for in vivo drug delivery. Small 2017, 13, 1602379.
Ang, C. Y.; Tan, S. Y.; Wang, X. L.; Zhang, Q.; Khan, M.; Bai, L. Y.; Tamil Selvan, S.; Ma, X.; Zhu, L. L.; Nguyen, K. T. et al. Supramolecular nanoparticle carriers self-assembled from cyclodextrin- and adamantane-functionalized polyacrylates for tumor-targeted drug delivery. J. Mater. Chem. B 2014, 2, 1879–1890.
Xu, X. Y.; Zeng, Z. S.; Chen, J.; Huang, B. Y.; Guan, Z. L.; Huang, Y. J.; Huang, Z. Q.; Zhao, C. S. Tumor-targeted supramolecular catalytic nanoreactor for synergistic chemo/chemodynamic therapy via oxidative stress amplification and cascaded Fenton reaction. Chem. Eng. J. 2020, 390, 124628.
Bai, Y.; Liu, C. P.; Chen, D.; Liu, C. F.; Zhuo, L. H.; Li, H.; Wang, C.; Bu, H. T.; Tian, W. β-Cyclodextrin-modified hyaluronic acid-based supramolecular self-assemblies for pH- and esterase- dual-responsive drug delivery. Carbohydr. Polym. 2020, 246, 116654.
Wankar, J.; Kotla, N. G.; Gera, S.; Rasala, S.; Pandit, A.; Rochev, Y. A. Recent advances in host-guest self-assembled cyclodextrin carriers: Implications for responsive drug delivery and biomedical engineering. Adv. Funct. Mater. 2020, 30, 1909049.
Parker, N.; Turk, M. J.; Westrick, E.; Lewis, J. D.; Low, P. S.; Leamon, C. P. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal. Biochem. 2005, 338, 284–293.
Ribeiro, D. T.; De Oliveira, R. C.; Mascio, P. D.; Menck, C. F. M. Singlet oxygen induces predominantly G to T transversions on a single-stranded shuttle vector replicated in monkey cells. Free Radic. Res. 1994, 21, 75–83.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Nos. 81703345 and 21974009), the Natural Science Foundation of Fujian Province (No. 2021J01549) and the National Health and Family Planning Commission Jointly established a scientific research fund (No. WKJ2016-2-14). We thank Servicebio Co., Ltd. (Wuhan, China) for technical support on tissue sections and fluorescent staining during in vivo experiments.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Yuan, G., Wang, Q., You, Z. et al. A novel hierarchical targeting and controllable smart nanoparticles for enhanced in situ nuclear photodynamic therapy. Nano Res. 15, 4212–4223 (2022). https://doi.org/10.1007/s12274-021-4027-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-021-4027-2