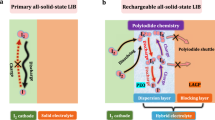
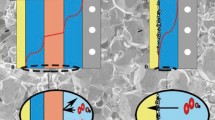
Abstract
Lithium-iodine (Li-I2) battery exhibits high potential to match with high-rate property and large energy density. However, problems of the system, such as evident sublimation of iodine elements, dissolution of iodine species in electrolyte, and lithium anode corrosion, prevent the practical use of rechargeable Li-I2 batteries. In this work, a molten Li-I2 typical cell design which has distinct advantages based on the solid-state garnet electrolyte with the eutectic iodate cathode is firstly developed. The U-shaped ceramic electrolyte tube can separate Li anode from the eutectic iodate cathode, so as to better tackle the above-mentioned inherent challenges for the liquid electrolyte systems. Without self-discharging and lithium anode corrosion, this solid-state battery system demonstrates high safety margin and excellent electrochemical performance. Also, the simple battery structure also indicates the easy assembly process and recycling of electrode materials. With the cathode loading of 593 mg in a single cell, an energy density of ∼ 506.7 Wh·kg−1 was achieved at 1 C and a long-term cycling life for 2,000 cycles also displays negligible capacity decay.

Similar content being viewed by others
References
Dunn, B.; Kamath, H.; Tarascon, J. M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935.
Larcher, D.; Tarascon J M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29.
Li, Z. H.; He, Q.; Zhou, C.; Li, Y.; Liu, Z. H.; Hong, X. F.; Xu, X.; Zhao, Y.; Mai, L. Rationally design lithiophilic surfaces toward high-energy lithium metal battery. Energy Stor. Mater. 2021, 37, 40–46.
Huang, K. S.; Bi, S.; Kurt, B.; Xu, C. Y.; Wu, L. Y.; Li, Z. W.; Feng, G.; Zhang, X. G. Regulation of SEI Formation by anion receptors to achieve ultra-stable lithium-metal batteries. Angew. Chem., Int. Ed. 2021, 23, 19232–19240.
Lin, X.; Yu, J.; Effat, M. B.; Zhou, G. D.; Robson, M. J.; Kwok, S. C. T.; Li, H. J.; Zhan, S. Y.; Shang, Y. L.; Ciucci, F. Ultrathin and non-flammable dual-salt polymer electrolyte for high-energy-density lithium-metal Battery. Adv. Funct. Mater. 2021, 31, 2010261.
Meng, Z.; Tan, X. J.; Zhang, S. L.; Ying, H. J.; Yan, X. F.; Tian, H. J.; Wang, G. X.; Han, W. Q. Ultra-stable binder-free rechargeable Li/I2 batteries enabled by “Betadine” chemical interaction. Chem. Commun. 2018, 54, 12337–12340.
Qiao, L.; Wang, C.; Zhao, X. S. Encapsulation of iodine in nitrogen-containing porous carbon plate arrays on carbon fiber cloth as a freestanding cathode for lithium-iodine batteries. ACS Appl. Energy Mater. 2021, 4, 7012–7019.
Ma, J. Z.; Liu, M. M.; He, Y. L.; Zhang, J. T. Iodine redox chemistry in rechargeable batteries. Angew. Chem., Int. Ed. 2021, 60, 12636–12647.
Zhao, X. Y.; Zhao-Karger, Z.; Fichtner, M.; Shen, X. D. Halide-based materials and chemistry for rechargeable batteries. Angew. Chem., Int. Ed. 2020, 132, 5902–5949.
Zhang, Q.; Zeng, Y. H.; Ye, S. H.; Liu, S. Inclusion complexation enhanced cycling performance of iodine/carbon composites for lithium-iodine battery. J. Power Sources 2020, 463, 228212.
Li, K. D.; Lin, B.; Li, Q. F.; Wang, H. F.; Zhang, S.; Deng, C. Anchoring iodine to N-doped hollow carbon fold-hemisphere: Toward a fast and stable cathode for rechargeable lithium-iodine batteries. ACS Appl. Mater. Interfaces 2017, 9, 20508–20518.
Sonigara, K. K.; Zhao, J. W.; Machhi, H. K.; Cui, G. L.; Soni, S. S. Self-assembled solid-state gel catholyte combating iodide diffusion and self-discharge for a stable flexible aqueous Zn-I2 battery. Adv. Energy Mater. 2020, 10, 2001997.
Tang, X.; Zhou, D.; Li, P.; Guo, X.; Wang, C. Y.; Kang, F. Y.; Li, B. H.; Wang, G. X. High-performance quasi-solid-state MXene-based Li-I batteries. ACS Cent. Sci. 2019, 5, 365–373.
Liu, F. C.; Liu, W. M.; Zhan, M. H.; Fu, Z. W.; Li, H. An all solid-state rechargeable lithium-iodine thin film battery using LiI (3-hydroxypropionitrile)2 as an I− ion electrolyte. Energy Environ. Sci 2011, 4, 1261–1264.
Li, K.; Chen, S.; Chen, S.; Liu, X. E.; Pan, W.; Zhang, J. T. Nitrogen, phosphorus co-doped carbon cloth as self-standing electrode for lithium-iodine batteries. Nano Res 2019, 12, 549–555.
Sun, C.; Shi, X. L.; Zhang, Y. B.; Liang, J. J.; Qu, J.; Lai, C. Ti3C2Tx MXene interface layer driving ultra-stable lithium-iodine batteries with both high iodine content and mass loading. ACS Nano 2020, 14, 1176–1184.
Liu, K.; Lang, J. L.; Yang, M. Z.; Xu, J.; Sun, B.; Wu, Y. L.; Wang, K. Y.; Zheng, Z. K.; Huang, Z. Y.; Wang, C. A. et al. Molten lithium-brass/zinc chloride system as high-performance and low-cost battery. Matter 2020, 3, 1714–1724.
Sun, B.; Liu, K.; Lang, J. L.; Fang, M. H.; Jin, Y.; Wu, H. Ionic liquid enabling stable interface in solid state lithium sulfur batteries working at room temperature. Electrochim. Acta 2018, 284, 662–668.
Sun, B.; Jin, Y.; Lang, J. L.; Liu, K.; Fang, M. H.; Wu, H. A painted layer for high-rate and high-capacity solid-state lithium-metal batteries. Chem. Commun. 2019, 55, 6704–6707.
Jin, Y.; Liu, K.; Lang, J. L.; Zhuo, D.; Huang, Z. Y.; Wang, C. A.; Wu, H.; Cui, Y. An intermediate temperature garnet-type solid electrolyte-based molten lithium battery for grid energy storage. Nat. Energy 2011, 3, 732–738.
Wu, Z. Z.; Xu, J. T.; Zhang, Q.; Wang, H. B.; Ye, S. H.; Wang, Y. L.; Lai, C. LiI embedded meso-micro porous carbon polyhedrons for lithium iodine battery with superior lithium storage properties. Energy Stor. Mater. 2011, 10, 62–68.
Tian, H. J.; Zhang, S. L.; Meng, Z.; He, W.; Han, W. Q. Rechargeable aluminum/iodine battery redox chemistry in ionic liquid electrolyte. ACS Energy Lett. 2017, 2, 1170–1176.
Lu, K.; Hu, Z. Y.; Ma, J. Z.; Ma, H. Y.; Dai, L. M.; Zhang, J. T. A rechargeable iodine-carbon battery that exploits ion intercalation and iodine redox chemistry. Nat. Commun. 2017, 8, 527.
Wang, F. X.; Liu, Z. C.; Yang, C. Q.; Zhong, H. X.; Nam, G.; Zhang, P. P.; Dong, R. H.; Wu, Y. P.; Cho, J.; Zhang, J. et al. Fully conjugated phthalocyanine copper metal-organic frameworks for sodium-iodine batteries with long-time-cycling durability. Adv. Mater. 2020, 32, 1905361.
Weppner, W.; Huggins, R. A. Determination of the kinetic parameters of mixed-conducting electrodes and application to the system Li3Sb. J. Electrochem. Soc. 1977, 124, 1569.
Shaju, K. M.; Rao, G. V. S.; Chowdari, B. V. R. EIS and GITT studies on oxide cathodes, O2-Li(2/3)+x (Co0.15Mn0.85) O2 (x = 0 and 1/3). Electrochim. Acta 2003, 48, 2691–2703.
Wu, S. L.; Zhang, W.; Song, X. Y.; Shukla, A. K.; Liu, G.; Battaglia, V.; Srinivasan, V. High rate capability of Li(Ni1/3Mn1/3Co1/3)O2 electrode for Li-ion batteries. J. Electrochem. Soc. 2012, 159, A438.
Cai, F. S.; Duan, Y. Q.; Yuan, Z. H. Iodine/β-cyclodextrin composite cathode for rechargeable lithium-iodine batteries. J. Mater. Sci. Mater. Electron. 2018, 29, 11540–11545.
Wang, H. F.; Zhang, G. M.; Ke, L. L.; Liu, B. D.; Zhang, S.; Deng, C. Understanding the effects of 3D porous architectures on promoting lithium or sodium intercalation in iodine/C cathodes synthesized via a biochemistry-enabled strategy. Nanoscale 2017, 9, 9365–9375.
Guo, Y. M.; Jiang, Y. L.; Zhang, Q.; Wan, D. Y.; Huang, C. Directional LiFePO4 cathode structure by freeze tape casting to improve lithium ion diffusion kinetics. J. Power Sources 2021, 506, 230052.
Zhao, J.; Shi, Q. L.; Xiang, Y. J.; Xia, Y. Y. Preparation of LiTi2O4 as a lithium-ion battery anode by a carbon-thermal reduction method. Int. J. Electrochem. Sci. 2018, 13, 1921–1930.
Chen, R. S.; Nolan, A. M.; Lu, J. Z.; Wang, J. Y.; Yu, X. Q.; Mo, Y. F.; Chen, L. Q.; Huang, X. J.; Li, H. The thermal stability of lithium solid electrolytes with metallic lithium. Joule 2020, 4, 812–821.
Acknowledgements
Research reported in this publication is supported by Zhengzhou University.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Sun, B., Wang, P., Xu, J. et al. A garnet-electrolyte based molten Li-I2 battery with high performance. Nano Res. 15, 4076–4082 (2022). https://doi.org/10.1007/s12274-021-4010-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-021-4010-y