Abstract
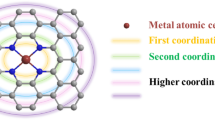
Designing highly efficient bifunctional electrocatalysts for oxygen reduction and evolution reaction (ORR/OER) is extremely important for developing regenerative fuel cells and metal-air batteries. Single-atom catalysts (SACs) have gained considerable attention in recent years because of their maximum atom utilization efficiency and tunable coordination environments. Herein, through density functional theory (DFT) calculations, we systematically explored the ORR/OER performances of nitrogen-coordinated transition metal carbon materials (TM-Nx-C (TM = Mn, Fe, Co, Ni, Cu, Pd, and Pt; x = 3, 4)) through tailoring the coordination environment. Our results demonstrate that compared to conventional tetra-coordinated (TM-N4-C) catalysts, the asymmetric tri-coordinated (TM-N3-C) catalysts exhibit stronger adsorption capacity of catalytic intermediates. Among them, Ni-N3-C possesses optimal adsorption energy and the lowest overpotential of 0.29 and 0.28 V for ORR and OER, respectively, making it a highly efficient bifunctional catalyst for oxygen catalysis. Furthermore, we find this enhanced effect stems from the additional orbital interaction between newly uncoordinated d-orbitals and p-orbitals of oxygenated species, which is evidently testified via the change of d-band center and integral crystal orbital Hamilton population (ICOHP). This work not only provides a potential bifunctional oxygen catalyst, but also enriches the knowledge of coordination engineering for tailoring the activity of SACs, which may pave the way to design and discover more promising bifunctional electrocatalysts for oxygen catalysis.

Similar content being viewed by others
References
Zhou, Y. N.; Gao, G. P.; Chu, W.; Wang, L. W. Transition-metal single atoms embedded into defective BC3 as efficient electrocatalysts for oxygen evolution and reduction reactions. Nanoscale 2021, 13, 1331–1339.
Wang, Y. W.; Qiu, W. J.; Song, E. H.; Gu, F.; Zheng, Z. H.; Zhao, X. L.; Zhao, Y. Q.; Liu, J. J.; Zhang, W. Q. Adsorption-energy-based activity descriptors for electrocatalysts in energy storage applications. Natl. Sci. Rev. 2018, 5, 327–341.
Wang, J.; Kong, H.; Zhang, J. Y.; Hao, Y.; Shao, Z. P.; Ciucci, F. Carbon-based electrocatalysts for sustainable energy applications. Prog. Mater. Sci. 2021, 116, 100717.
Huang, X. Q.; Zhao, Z. P.; Cao, L.; Chen, Y.; Zhu, E. B.; Lin, Z. Y.; Li, M. F.; Yan, A. M.; Zettl, A.; Wang, Y. M. et al. Highperformance transition metal-doped Pt3Ni octahedra for oxygen reduction reaction. Science 2015, 348, 1230–1234.
Frydendal, R.; Paoli, E. A.; Knudsen, B. P.; Wickman, B.; Malacrida, P.; Stephens, I. E. L.; Chorkendorff, I. Benchmarking the stability of oxygen evolution reaction catalysts: The importance of monitoring mass losses. ChemElectroChem 2014, 1, 2075–2081.
Lee, Y.; Suntivich, J.; May, K. J.; Perry, E. E.; Shao-Horn, Y. Synthesis and activities of rutile IrO2 and RuO2 nanoparticles for oxygen evolution in acid and alkaline solutions. J. Phys. Chem. Lett. 2012, 3, 399–404.
Chen, J. Y.; Lim, B.; Lee, E. P.; Xia, Y. N. Shape-controlled synthesis of platinum nanocrystals for catalytic and electrocatalytic applications. Nano Today 2009, 4, 81–95.
Zhao, Y. X.; Zhang, S.; Shi, R.; Waterhouse, G. I. N.; Tang, J. W.; Zhang, T. R. Two-dimensional photocatalyst design: A critical review of recent experimental and computational advances. Mater. Today 2020, 34, 78–91.
Wang, B.; Wu, X. Y.; Zhang, X. Y.; Pang, G. G.; Li, S. M. Mo2C-embedded biomass-derived honeycomb-like nitrogen-doped carbon nanosheet/graphene aerogel films for highly efficient electrocatalytic hydrogen evolution. New J. Chem. 2020, 44, 1147–1156.
Liu, Z.; Wang, J. Q.; Zhan, C. H.; Yu, J.; Cao, Y.; Tu, J. C.; Shi, C. S. Phosphide-oxide honeycomb-like heterostructure CoP@CoMoO4/CC for enhanced hydrogen evolution reaction in alkaline solution. J. Mater. Sci. Technol. 2020, 46, 177–184.
Choi, W. S.; Jang, M. J.; Park, Y. S.; Lee, K. H.; Lee, J. Y.; Seo, M. H.; Choi, S. M. Three-dimensional honeycomb-like Cu0.81Co2.19O4 nanosheet arrays supported by Ni foam and their high efficiency as oxygen evolution electrodes. ACS Appl. Mater. Interface s 2018, 10, 38663–38668.
Chen, Z. J.; Cao, G. X.; Gan, L. Y.; Dai, H.; Xu, N.; Zang, M. J.; Dai, H. B.; Wu, H.; Wang, P. Highly dispersed platinum on honeycomb-like NiO@Ni film as a synergistic electrocatalyst for the hydrogen evolution reaction. ACS Catal. 2018, 8, 8866–8872.
Long, X.; Li, G. X.; Wang, Z. L.; Zhu, H. Y.; Zhang, T.; Xiao, S.; Guo, W. Y.; Yang, S. H. Metallic iron-nickel sulfide ultrathin nanosheets as a highly active electrocatalyst for hydrogen evolution reaction in acidic media. J. Am. Chem. Soc. 2015, 137, 11900–11903.
Zitolo, A.; Goellner, V.; Armel, V.; Sougrati, M. T.; Mineva, T.; Stievano, L.; Fonda, E.; Jaouen, F. Identification of catalytic sites for oxygen reduction in iron- and nitrogen-doped graphene materials. Nat. Mater. 2015, 14, 937–942.
Chen, L. Y.; Zhang, L.; Chen, Z. J.; Liu, H. L.; Luque, R.; Li, Y. W. A covalent organic framework-based route to the in situ encapsulation of metal nanoparticles in N-rich hollow carbon spheres. Chem. Sci. 2016, 7, 6015–6020.
Fan, L. L.; Liu, P. F.; Yan, X. C.; Gu, L.; Yang, Z. Z.; Yang, H. G.; Qiu, S. L.; Yao, X. D. Atomically isolated nickel species anchored on graphitized carbon for efficient hydrogen evolution electrocatalysis. Nat. Commun. 2016, 7, 10667.
Cao, Y. J.; Chen, S.; Luo, Q. Q.; Yan, H.; Lin, Y.; Liu, W.; Cao, L. L.; Lu, J. L.; Yang, J. L.; Yao, T. et al. Atomic-level insight into optimizing the hydrogen evolution pathway over a Co1-N4 single-site photocatalyst. Angew. Chem., Int. Ed. 2017, 56, 12191–12196.
Zhao, D.; Zhuang, Z. W.; Cao, X.; Zhang, C.; Peng, Q.; Chen, C.; Li, Y. D. Atomic site electrocatalysts for water splitting, oxygen reduction and selective oxidation. Chem. Soc. Re v. 2020, 49, 2215–2264.
Chen, G. B.; Liu, P.; Liao, Z. Q.; Sun, F. F.; He, Y. H.; Zhong, H. X.; Zhang, T.; Zschech, E.; Chen, M. W.; Wu, G. et al. Zinc-mediated template synthesis of Fe-N-C electrocatalysts with densely accessible Fe-Nx active sites for efficient oxygen reduction. Adv. Mater. 2020, 32, 1907399.
Wang, Y.; Mao, J.; Meng, X. G.; Yu, L.; Deng, D. H.; Bao, X. H. Catalysis with two-dimensional materials confining single atoms: Concept, design, and applications. Chem. Re v. 2019, 119, 1806–1854.
Wang, J.; Jia, L. J.; Zhong, J.; Xiao, Q. B.; Wang, C.; Zang, K. T.; Liu, H. T.; Zheng, H. C.; Luo, J.; Yang, J. et al. Single-atom catalyst boosts electrochemical conversion reactions in batteries. E nergy Storage Mater. 2019, 18, 246–252.
Hong, J. H.; Jin, C. H.; Yuan, J.; Zhang, Z. Atomic defects in two-dimensional materials: From single-atom spectroscopy to functionalities in opto-/electronics, nanomagnetism, and catalysis. A dv. Mater. 2017, 29, 1606434.
Xie, X. Y.; Peng, L. S.; Yang, H. Z.; Waterhouse, G. I. N.; Shang, L.; Zhang, T. R. MIL-101-derived mesoporous carbon supporting highly exposed Fe single-atom sites as efficient oxygen reduction reaction catalysts. Adv. Mater. 2021, 33, 2101038.
Zhao, L.; Zhang, Y.; Huang, L. B.; Liu, X. Z.; Zhang, Q. H.; He, C.; Wu, Z. Y.; Zhang, L. J.; Wu, J. P.; Yang, W. L. et al. Cascade anchoring strategy for general mass production of high-loading single-atomic metal-nitrogen catalysts. Nat. Commun. 2019, 10, 1278.
Jiao, L.; Zhang, R.; Wan, G.; Yang, W. J.; Wan, X.; Zhou, H.; Shui, J. L.; Yu, S. H.; Jiang, H. L. Nanocasting SiO2 into metal-organic frameworks imparts dual protection to high-loading Fe single-atom electrocatalysts. Nat. Commun. 2020, 11, 2831.
Chen, R. R.; Li, H. X.; Chu, D.; Wang, G. F. Unraveling oxygen reduction reaction mechanisms on carbon-supported Fe-phthalocyanine and Co-phthalocyanine catalysts in alkaline solutions. J. Phys. Chem. C 2009, 113, 20689–20697.
Kattel, S.; Atanassov, P.; Kiefer, B. Catalytic activity of Co-Nx/C electrocatalysts for oxygen reduction reaction: A density functional theory study. Phys. Chem. Chem. Phys. 2013, 15, 148–153.
Calle-Vallejo, F.; Martínez, J. I.; Rossmeisl, J. Density functional studies of functionalized graphitic materials with late transition metals for oxygen reduction reactions. Phys. Chem. Chem. Phy s. 2011, 13, 15639–15643.
Beaumier, E. P.; Pearce, A. J.; See, X. Y.; Tonks, I. A. Modern applications of low-valent early transition metals in synthesis and catalysis. Nat. Rev. Chem. 2019, 3, 15–34.
Burford, R. J.; Yeo, A.; Fryzuk, M. D. Dinitrogen activation by group 4 and group 5 metal complexes supported by phosphine-amido containing ligand manifolds. Coord. Chem. Rev. 2017, 334, 84–99.
Zhang, X. Y.; Zhang, S.; Yang, Y.; Wang, L. G.; Mu, Z. J.; Zhu, H. S.; Zhu, X. Q.; Xing, H. H.; Xia, H. Y.; Huang, B. L. et al. A general method for transition metal single atoms anchored on honeycomblike nitrogen-doped carbon nanosheets. Adv. Mate r. 2020, 32, 1906905.
Lin, Y. C.; Liu, P. Y.; Velasco, E.; Yao, G.; Tian, Z. Q.; Zhang, L. J.; Chen, L. Fabricating single-atom catalysts from chelating metal in open frameworks. Adv. Mater. 2019, 31, 1808193.
Chen, Z. G.; Gong, W. B.; Liu, Z. B.; Cong, S.; Zheng, Z. H.; Wang, Z.; Zhang, W.; Ma, J. Y.; Yu, H. S.; Li, G. H. et al. Coordination-controlled single-atom tungsten as a non-3d-metal oxygen reduction reaction electrocatalyst with ultrahigh mass activity. Nano Energy 2019, 60, 394–403.
Chen, Y. J.; Ji, S. F.; Zhao, S.; Chen, W. X.; Dong, J. C.; Cheong, W. C.; Shen, R. A.; Wen, X. D.; Zheng, L. R.; Rykov, A. I. et al. Enhanced oxygen reduction with single-atomic-site iron catalysts for a zinc-air battery and hydrogen-air fuel cell. Nat. Commun. 2018, 9, 5422.
Gong, L. L.; Wang, X. W.; Zheng, T.; Liu, J.; Wang, J.; Yang, Y. C.; Zhang, J.; Han, X.; Zhang, L. P.; Xia, Z. H. Catalytic mechanism and design principle of coordinately unsaturated single metal atom-doped covalent triazine frameworks with high activity and selectivity for CO2 electroreduction. J. Mater. Chem. A 2021, 9, 3555–3566.
Zeng, X. J.; Shui, J. L.; Liu, X. F.; Liu, Q. T.; Li, Y. C.; Shang, J. X.; Zheng, L. R.; Yu, R. H. Single-atom to single-atom grafting of Pt1 onto Fe-N4 center: Pt1@Fe-N-C multifunctional electrocatalyst with significantly enhanced properties. Adv. Energy Mate r. 2018, 8, 1701345.
Yang, Z. K.; Chen, B. X.; Chen, W. X.; Qu, Y. T.; Zhou, F. Y.; Zhao, C. M.; Xu, Q.; Zhang, Q. H.; Duan, X. Z.; Wu, Y. Directly transforming copper (I) oxide bulk into isolated single-atom copper sites catalyst through gas-transport approach. Nat. Commun. 2019, 1 0, 3734.
Zhang, Y.; Jiao, L.; Yang, W. J.; Xie, C. F.; Jiang, H. L. Rational fabrication of low-coordinate single-atom Ni electrocatalysts by MOFs for highly selective CO2 reduction. Angew. Chem., Int. E d. 2021, 60, 7607–7611.
Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186.
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 1994, 5 0, 17953–17979.
Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775.
Perdew, J. P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868.
Liao, X. B.; Lu, R. H.; Xia, L. X.; Liu, Q.; Wang, H.; Zhao, K.; Wang, Z. Y.; Zhao, Y. Density functional theory for electrocatalysis. Energy Environ. Mater., in press, DOI: https://doi.org/10.1002/eem2.12204.
Luo, S. J.; Zhao, Y.; Truhlar, D. G. Improved CO adsorption energies, site preferences, and surface formation energies from a meta-generalized gradient approximation exchange-correlation functional, M06-L. J. Phys. Chem. Lett. 2012, 3, 2975–2979.
Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 1 32, 154104.
Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799.
Tang, W.; Sanville, E.; Henkelman, G. A grid-based Bader analysis algorithm without lattice bias. J. Phys.: Condens. Matte r 2009, 21, 084204.
Maintz, S.; Deringer, V. L.; Tchougreeff, A. L.; Dronskowski, R. LOBSTER: A tool to extract chemical bonding from plane-wave based DFT. J. Comput. Chem. 2016, 37, 1030–1035.
Deringer, V. L.; Tchougréeff, A. L.; Dronskowski, R. Crystal orbital Hamilton population (COHP) analysis as projected from plane-wave basis sets. J. Phys. Chem. A 2011, 115, 5461–5466.
Mathew, K.; Sundararaman, R.; Letchworth-Weaver, K.; Arias, T. A.; Hennig, R. G. Implicit solvation model for density-functional study of nanocrystal surfaces and reaction pathways. J. Chem. Phys. 2014, 140, 084106.
Zhou, Y. N.; Gao, G. P.; Li, Y.; Chu, W.; Wang, L. W. Transition-metal single atoms in nitrogen-doped graphenes as efficient active centers for water splitting: A theoretical study. Phys. Chem. Chem. Phys. 2019, 21, 3024–3032.
Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511–519.
Niu, H.; Wang, X. T.; Shao, C.; Zhang, Z. F.; Guo, Y. Z. Computational screening single-atom catalysts supported on g-CN for N2 reduction: High activity and selectivity. ACS Sustainabl e Chem. Eng. 2020, 8, 13749–13758.
Rossmeisl, J.; Logadottir, A.; Nørskov, J. K. Electrolysis of water on (oxidized) metal surfaces. Chem. Phys. 2005, 319, 178–184.
Nørskov, J. K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J. R.; Bligaard, T.; Jónsson, H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 2004, 108, 17886–17892.
Niu, H.; Wan, X. H.; Wang, X. T.; Shao, C.; Robertson, J.; Zhang, Z. F.; Guo, Y. Z. Single-atom rhodium on defective g-C3N4: A promising bifunctional oxygen electrocatalyst. ACS Sustainabl e Chem. Eng. 2021, 9, 3590–3599.
Xu, H. X.; Cheng, D. J.; Cao, D. P.; Zeng, X. C. A universal principle for a rational design of single-atom electrocatalysts. Nat. Catal. 2018, 1, 339–348.
Impeng, S.; Junkaew, A.; Maitarad, P.; Kungwan, N.; Zhang, D. S.; Shi, L. Y.; Namuangruk, S. A MnN4 moiety embedded graphene as a magnetic gas sensor for CO detection: A first principle study. Appl. Surf. Sci. 2019, 473, 820–827.
Zhang, X. L.; Yang, Z. X.; Lu, Z. S.; Wang, W. C. Bifunctional CoNx embedded graphene electrocatalysts for OER and ORR: A theoretical evaluation. Carbon 2018, 130, 112–119.
Kattel, S.; Wang, G. F. Reaction pathway for oxygen reduction on FeN4 embedded graphene. J. Phys. Chem. Lett. 2014, 5, 452–456.
Xue, Z.; Zhang, X. Y.; Qin, J. Q.; Liu, R. P. TMN4 complex embedded graphene as bifunctional electrocatalysts for high efficiency OER/ORR. J. Energy Chem. 2021, 55, 437–443.
Deng, Q. M.; Han, J.; Zhao, J.; Chen, G. B.; Vegge, T.; Hansen, H. A. 1D metal-dithiolene wires as a new class of bi-functional oxygen reduction and evolution single-atom electrocatalysts. J. Catal. 2021, 3 93, 140–148.
Yang, J. R.; Li, W. H.; Wang, D. S.; Li, Y. D. Electronic metal-support interaction of single-atom catalysts and applications in electrocatalysis. Adv. Mater. 2020, 32, 2003300.
Nørskov, J. K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J. R.; Bligaard, T.; Jónsson, H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 2004, 108, 17886–17892.
Rossmeisl, J.; Qu, Z. W.; Zhu, H.; Kroes, G. J.; Nørskov, J. K. Electrolysis of water on oxide surfaces. J. Electroanal. Chem. 2007, 6 07, 83–89.
Medford, A. J.; Vojvodic, A.; Hummelshøj, J. S.; Voss, J.; Abild-Pedersen, F.; Studt, F.; Bligaard, T.; Nilsson, A.; Nørskov, J. K. From the Sabatier principle to a predictive theory of transition-metal heterogeneous catalysis. J. Catal. 2015, 328, 36–42.
Zhang, Z. H.; Qi, S. Y.; Song, X. H.; Wang, J.; Zhang, W. Q.; Zhao, M. W. Stable multifunctional single-atom catalysts adsorbed on pyrazine-modified graphyne. Appl. Surf. Sci. 2021, 553, 149464.
Li, J.; Chen, S. G.; Yang, N.; Deng, M. M.; Ibraheem, S.; Deng, J. H.; Li, J.; Li, L.; Wei, Z. D. Ultrahigh-loading zinc single-atom catalyst for highly efficient oxygen reduction in both acidic and alkaline media. Angew. Chem., Int. Ed. 2019, 58, 7035–7039.
Persson, K. A.; Waldwick, B.; Lazic, P.; Ceder, G. Prediction of solid-aqueous equilibria: Scheme to combine first-principles calculations of solids with experimental aqueous states. Phys. Rev. B 2012, 85, 235438.
Mao, X.; Ling, C. Y.; Tang, C.; Yan, C.; Zhu, Z. H.; Du, A. J. Predicting a new class of metal-organic frameworks as efficient catalyst for bi-functional oxygen evolution/reduction reactions. J. Catal. 2018, 367, 206–211.
Ling, C. Y.; Shi, L.; Ouyang, Y. X.; Zeng, X. C.; Wang, J. L. Nanosheet supported single-metal atom bifunctional catalyst for overall water splitting. Nano Lett. 2017, 17, 5133–5139.
Fu, Z. Z.; Ling, C. Y.; Wang, J. L. A Ti3C2O2 supported single atom, trifunctional catalyst for electrochemical reactions. J. Mater. Chem. A 2020, 8, 7801–7807.
Shang, H. S.; Zhou, X. Y.; Dong, J. C.; Li, A.; Zhao, X.; Liu, Q. H.; Lin, Y.; Pei, J. J.; Li, Z.; Jiang, Z. L. et al. Engineering unsymmetrically coordinated Cu-S1N3 single atom sites with enhanced oxygen reduction activity. Nat. Commun. 2020, 11, 3049.
Acknowledgements
We thank the following funding agencies for supporting this work: Foshan Xianhu Laboratory of the Advanced Energy Science and Technology Guangdong Laboratory (No. XHT2020-003), the China Postdoctoral Science Foundation (No. 2021M692490), and the Fundamental Research Funds for the Central Universities (No. WUT:2020III029, 2020IVA100).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Xiao, G., Lu, R., Liu, J. et al. Coordination environments tune the activity of oxygen catalysis on single atom catalysts: A computational study. Nano Res. 15, 3073–3081 (2022). https://doi.org/10.1007/s12274-021-3964-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-021-3964-0