Abstract
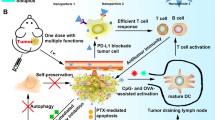
The therapeutic efficacy of programmed cell death protein 1/programmed cell death-ligand 1 (PD-1/PD-L1) blockade immunotherapy is extremely dampened by complex immunosuppressive mechanisms including regulatory T cells (Treg), M2 macrophages (M2), and prostaglandin E2 (PGE2). The pivotal roles of PGE2 have been recognized by directly inactivating CD8+ T cells and indirectly inducing Treg and M2. Therefore, PGE2 abolishment through inactivating cyclooxygenase-2 (COX-2) could be robust to sensitize tumour toward anti-PD-1/PD-L1 immunotherapy, which has gone into clinical trials. However, exploring this promising strategy in nanomedicine to enhance immunotherapy remains unrevealed. The key challenge to synergistically combine COX-2 inhibition and anti-PD-1/PD-L1 lies in the different pharmacokinetic profiles and the spatial obstacles since PD-1/PD-L1 interaction occurs extracellularly and COX-2 locates intracellularly. Thus, the programmed release nanoparticles (termed as Cele-BMS-NPs) are rationally designed, which are composed of pH-sensitive human serum albumin derivative, BMS-202 compound as PD-1/PD-L1 inhibitor, glutathione (GSH)-activatable prodrug of celecoxib (COX-2 inhibitor). The in vitro experiments demonstrate that this smart Cele-BMS-NPs could extracellularly release BMS-202 under the acidic tumour microenvironment, and the intracellularly release of celecoxib in response to the elevated GSH concentration inside tumour cells. After systemic administration, the intratumoral infiltration of CD8+ T cells is significantly enhanced and meanwhile immunosuppressive M2, Treg, and PGE2 are reduced, thereby eliciting the anti-tumour immune responses toward low immunogenic tumours and postsurgical tumour recurrences.

Similar content being viewed by others
References
Couzin-Frankel, J. Cancer immunotherapy. Science 2013, 342, 1432–1433.
Robert, C.; Schachter, J.; Long, G. V.; Arance, A.; Grob, J. J.; Mortier, L.; Daud, A.; Carlino, M. S.; McNeil, C.; Lotem, M. et al. Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl. J. Med. 2015, 372, 2521–2532.
Sharma, P.; Allison, J. P. The future of immune checkpoint therapy. Science 2015, 348, 56–61.
Weber, J. S.; D’Angelo, S. P.; Minor, D.; Hodi, F. S.; Gutzmer, R.; Neyns, B.; Hoeller, C.; Khushalani, N. I.; Miller, W. H. Jr.; Lao, C. D. et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015, 16, 375–384.
Brahmer, J. R.; Tykodi, S. S.; Chow, L. Q. M; Hwu, W. J.; Topalian, S. L.; Hwu, P.; Drake, C. G.; Camacho, L. H.; Kauh, J.; Odunsi, K. et al. Safety and activity of anti-PD-Ll antibody in patients with advanced cancer. N. Engl. J. Med. 2012, 366, 2455–2465.
Kim, K.; Skora, A. D.; Li, Z. B.; Liu, Q.; Tam, A. J.; Blosser, R. L.; Diaz, L. A. Jr.; Papadopoulos, N.; Kinzler, K. W.; Vogelstein, B. et al. Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid-derived cells. Proc. Natl. Acad. Sci. USA 2014, 111, 11774–11779.
Postow, M. A.; Sidlow, R.; Hellmann, M. D. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 2018, 378, 158–168.
Lybaert, L.; Vermaelen, K.; De Geest, B. G.; Nuhn, L. Immunoengineering through cancer vaccines—a personalized and multi-step vaccine approach towards precise cancer immunity. J. Control. Release 2018, 289, 125–145.
Nuhn, L.; De Koker, S.; Van Lint, S.; Zhong, Z. F.; Catani, J. P.; Combes, F.; Deswarte, K.; Li, Y. P.; Lambrecht, B. N.; Lienenklaus, S. et al. Nanoparticle-conjugate TLR7/8 agonist localized immunotherapy provokes safe antitumoral responses. Adv. Mater. 2018, 45, 1803397.
Butt, A. Q.; Mills, K. H. G. Immunosuppressive networks and checkpoints controlling antitumor immunity and their blockade in the development of cancer immunotherapeutics and vaccines. Oncogene 2014, 33, 4623–4631.
Adams, J. L.; Smothers, J.; Srinivasan, R.; Hoos, A. Big opportunities for small molecules in immuno-oncology. Nat. Rev. Drug Discov. 2015, 14, 603–622.
Kryczek, I.; Wei, S.; Zou, L. H.; Zhu, G. F.; Mottram, P.; Xu, H. B.; Chen, L. P.; Zou, W. P. Cutting edge: Induction of B7-H4 on APCs through IL-10: Novel suppressive mode for regulatory T cells. J. Immunol. 2006, 177, 40–44.
Nakanishi, Y.; Nakatsuji, M.; Seno, H.; Ishizu, S.; Akitake-Kawano, R.; Kanda, K.; Ueo, T.; Komekado, H.; Kawada, M.; Minami, M. et al. COX-2 inhibition alters the phenotype of tumor-associated macrophages from M2 to M1 in ApcMin/+ mouse polyps. Carcinogenesis 2011, 32, 1333–1339.
Ruffell, B.; Affara, N. I.; Coussens, L. M. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012, 33, 119–126.
Noy, R.; Pollard, J. W. Tumor-associated macrophages: From mechanisms to therapy. Immunity 2014, 41, 49–61.
Mahic, M.; Yaqub, S.; Johansson, C. C.; Taskén, K.; Aandahl, E. M. FOXP3+CD4+CD25+ adaptive regulatory T cells express cyclooxygenase-2 and suppress effector T cells by a prostaglandin E2-dependent mechanism. J. Immunol. 2006, 177, 246–254.
Wang, S. J.; Khullar, K.; Yegya-Raman, N.; Kim, S.; Silk, A. W.; Malhotra, J.; Gentile, M. A.; Mehnert, J. M.; Jabbour, S. K. Cyclooxygenase inhibitor use during checkpoint blockade immunotherapy and effect on time to progression for metastatic melanoma patients. J. Clin. Oncol. 2019, 37, e21029.
Wang, S. J.; Khullar, K.; Kim, S.; Yegya-Raman, N.; Malhotra, J.; Groisberg, R.; Crayton, S. H.; Silk, A. W.; Nosher, J. L.; Gentile, M. A. et al. Effect of cyclo-oxygenase inhibitor use during checkpoint blockade immunotherapy in patients with metastatic melanoma and non-small cell lung cancer. J. ImmunoTher. Cancer 2020, 8, e000889.
Yang, J. X.; Wang, C. H.; Shi, S.; Dong, C. Y. Nanotechnologies for enhancing cancer immunotherapy. Nano Res. 2020, 13, 2595–2616.
Li, Z. L.; Hu, Y.; Miao, Z. H.; Xu, H.; Li, C. X.; Zhao, Y.; Li, Z.; Chang, M. L.; Ma, Z.; Sun, Y. et al. Dual-stimuli responsive bismuth nanoraspberries for multimodal imaging and combined cancer therapy. Nano Lett. 2018, 18, 6778–6788.
Rajendrakumar, S. K.; Cherukula, K.; Park, H. J.; Uthaman, S.; Jeong, Y. Y.; Lee, B. I.; Park, I. K. Dual-stimuli-responsive albumin-polyplex nanoassembly for spatially controlled gene release in metastatic breast cancer. J. Control. Release 2018, 276, 72–83.
Qu, Y.; Chu, B. Y.; Wei, X. W.; Lei, M. Y.; Hu, D. R.; Zha, R. Y.; Zhong, L.; Wang, M. Y.; Wang, F. F.; Qian, Z. Y. Redox/pH dual-stimuli responsive camptothecin prodrug nanogels for “on-demand” drug delivery. J. Control. Release 2019, 296, 93–106.
Zhang, X. L.; Zhang, C. N.; Cheng, M. B.; Zhang, Y. H.; Wang, W.; Yuan, Z. Dual pH-responsive “charge-reversal like” gold nanoparticles to enhance tumor retention for chemo-radiotherapy. Nano Res. 2019, 12, 2815–2856.
Zhang, Z. Z.; Wang, Q. X.; Liu, Q.; Zheng, Y. D.; Zheng, C. X.; Yi, K. K.; Zhao, Y.; Gu, Y.; Wang, Y.; Wang, C. et al. Dual-locking nanoparticles disrupt the PD-1/PD-L1 pathway for efficient cancer immunotherapy. Adv. Mater. 2019, 31, 1905751.
Liu, J. Y.; Liu, W. G.; Weitzhandler, I.; Bhattacharyya, J.; Li, X. H.; Wang, J.; Qi, Y. Z.; Bhattacharjee, S.; Chilkoti, A. Ring-opening polymerization of prodrugs: A versatile approach to prepare well-defined drug-loaded nanoparticles. Angew. Chem., Int. Ed. 2015, 54, 1002–1006.
Noh, J.; Kwon, B.; Han, E.; Park, M.; Yang, W.; Cho, W.; Yoo, W.; Khang, G.; Lee, D. Amplification of oxidative stress by a dual stimuli-responsive hybrid drug enhances cancer cell death. Nat. Commun. 2015, 6, 6907.
Feng, L. D.; Wang, Y. Q.; Luo, Z. L.; Huang, Z.; Zhang, Y.; Guo, K.; Ye, D. J. Dual stimuli-responsive nanoparticles for controlled release of anticancer and anti-inflammatory drugs combination. Chem.-Eur. J. 2017, 23, 9397–9406.
Palanikumar, L.; Jeena, M. T.; Kim, K.; Oh, J. Y.; Kim, C.; Park, M. H.; Ryu, J. H. Spatiotemporally and sequentially-controlled drug release from polymer gatekeeper-hollow silica nanoparticles. Sci. Rep. 2017, 7, 46540.
Sun, Z. Q.; Liu, G. H.; Hu, J. M.; Liu, S. Y. Photo- and reduction-responsive polymersomes for programmed release of small and macromolecular payloads. Biomacromolecules 2018, 19, 2071–2081.
Webb, B. A.; Chimenti, M.; Jacobson, M. P.; Barber, D. L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677.
Eisele, K.; Gropeanu, R. A.; Zehendner, C. M.; Rouhanipour, A.; Ramanathan, A.; Mihov, G.; Koynov, K.; Kuhlmann, C. R. W.; Vasudevan, S. G.; Luhmann, H. J. et al. Fine-tuning DNA/albumin polyelectrolyte interactions to produce the efficient transfection agent cBSA-147. Biomaterials 2010, 31, 8789–8801.
Wu, Y. Z.; Pramanik, G.; Eisele, K.; Weil, T. Convenient approach to polypeptide copolymers derived from native proteins. Biomacromolecules 2012, 13, 1890–1898.
Wu, Y. Z.; Ihme, S.; Feuring-Buske, M.; Kuan, S. L.; Eisele, K.; Lamla, M.; Wang, Y. R.; Buske, C.; Weil, T. A core-shell albumin copolymer nanotransporter for high capacity loading and two-step release of doxorubicin with enhanced anti-leukemia activity. Adv. Healthc. Mater. 2013, 2, 884–894.
Meier, C.; Wu, Y. Z.; Pramanik, G.; Weil, T. Self-assembly of high molecular weight polypeptide copolymers studied via diffusion limited aggregation. Biomacromolecules 2014, 15, 219–227.
Wu, Y. Z.; Ermakova, A.; Liu, W. N.; Pramanik, G.; Vu, T. M.; Kurz, A.; McGuinness, L.; Naydenov, B.; Hafner, S.; Reuter, R. et al. Programmable biopolymers for advancing biomedical applications of fluorescent nanodiamonds. Adv. Funct. Mater. 2015, 25, 6576–6585.
Alouane, A.; Labruère, R.; Le Saux, T.; Schmidt, F.; Jullien, L. Self-immolative spacers: Kinetic aspects, structure-property relationships, and applications. Angew. Chem., Int. Ed. 2015, 54, 7492–7509.
Roth, M. E.; Green, O.; Gnaim, S.; Shabat, D. Dendritic, oligomeric, and polymeric self-immolative molecular amplification. Chem. Rev. 2016, 116, 1309–1352.
Boussif, O.; Zanta, M. A.; Behr, J. P. Optimized galenics improve in vitro gene transfer with cationic molecules up to 1000-fold. Gene Ther. 1996, 3, 1074–1080.
Akinc, A.; Thomas, M.; Klibanov, A. M.; Langer, R. Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. J. Gene Med. 2005, 7, 657–663.
Jiang, Y. Y.; Lu, H. X.; Dag, A.; Hart-Smith, G.; Stenzel, M. H. Albumin-polymer conjugate nanoparticles and their interactions with prostate cancer cells in 2D and 3D culture: Comparison between PMMA and PCL. J. Mater. Chem B 2016, 4, 2017–2027.
Jiang, Y. Y.; Wong, S.; Chen, F.; Chang, T.; Lu, H. X.; Stenzel, M. H. Influencing selectivity to cancer cells with mixed nanoparticles prepared from albumin-polymer conjugates and block copolymers. Bioconjug. Chem. 2017, 28, 979–985.
Taguchi, K.; Lu, H. X.; Jiang, Y. Y.; Hung, T. T.; Stenzel, M. H. Safety of nanoparticles based on albumin-polymer conjugates as a carrier of nucleotides for pancreatic cancer therapy. J. Mater. Chem. B 2018, 6, 6278–6287.
Piloni, A.; Wong, C. K.; Chen, F.; Lord, M.; Walther, A.; Stenzel, M. H. Surface roughness influences the protein corona formation of glycosylated nanoparticles and alter their cellular uptake. Nanoscale 2019, 11, 23259–23267.
Ko, J. Y.; Park, S.; Lee, H.; Koo, H.; Kim, M. S.; Choi, K.; Kwon, I. C.; Jeong, S. Y.; Kim, K.; Lee, D. S. pH-sensitive nanoflash for tumoral acidic pH imaging in live animals. Small 2010, 6, 2539–2544.
Zhou, K. J.; Wang, Y. G.; Huang, X. N.; Luby-Phelps, K.; Sumer, B. D.; Gao, J. M. Tunable, ultrasensitive pH-responsive nanoparticles targeting specific endocytic organelles in living cells. Angew. Chem., Int. Ed. 2011, 50, 6109–6114.
Zhou, K. J.; Liu, H. M.; Zhang, S. R.; Huang, X. N.; Wang, Y. G.; Huang, G.; Sumer, B. D.; Gao, J. M. Multicolored pH-tunable and activatable fluorescence nanoplatform responsive to physiologic pH stimuli. J. Am. Chem. Soc. 2012, 134, 7803–7811.
Wang, Y. G.; Zhou, K. J.; Huang, G.; Hensley, C.; Huang, X. N.; Ma, X. P.; Zhao, T.; Sumer, B. D.; DeBerardinis, R. J.; Gao, J. M. A nanoparticle-based strategy for the imaging of a broad range of tumours by nonlinear amplification of microenvironment signals. Nat. Mater. 2014, 13, 204–212.
Guzik, K.; Zak, K. M.; Grudnik, P.; Magiera, K.; Musielak, B.; Törner, R.; Skalniak, L.; Dömling, A.; Dubin, G.; Holak, T. A. Small-molecule inhibitors of the programmed cell death-1/programmed death-ligand 1 (PD-1/PD-L1) interaction via transiently induced protein states and dimerization of PD-L1. J. Med. Chem. 2017, 60, 5857–5867.
Skalniak, L.; Zak, K. M.; Guzik, K.; Magiera, K.; Musielak, B.; Pachota, M.; Szelazek, B.; Kocik, J.; Grudnik, P.; Tomala, M. et al. Small-molecule inhibitors of PD-1/PD-L1 immune checkpoint alleviate the PD-L1-induced exhaustion of T-cells. Oncotarget 2017, 8, 72167–72181.
Konstantinidou, M.; Zarganes-Tzitzikas, T.; Magiera-Mularz, K.; Holak, T. A.; Dömling, A. Immune checkpoint PD-1/PD-L1: Is there life beyond antibodies? Angew. Chem., Int. Ed. 2018, 57, 4840–4848.
Wang, C.; Sun, W. J.; Wright, G.; Wang, A. Z.; Gu, Z. Inflammation-triggered cancer immunotherapy by programmed delivery of CpG and anti-PD1 antibody. Adv. Mater. 2016, 28, 8912–8920.
Wang, C.; Sun, W. J.; Ye, Y. Q.; Hu, Q. Y.; Bomba, H. N.; Gu, Z. In situ activation of platelets with checkpoint inhibitors for post-surgical cancer immunotherapy. Nat. Biomed. Eng. 2017, 1, 0011.
Acknowledgements
We acknowledge the financial support from The National Key R&D Program of China (No. 2018YFA0903500), The Postdoctoral Science Fund of China (No. 2017M622429), The National Natural Science Foundation of China (No. 51703073), and The 1000 Young Talent Program of China. We also thank the Analytical and Testing Centre of HUST, Analytical and Testing Centre of School of Chemistry and Chemical Engineering (HUST), and Research Core Facilities for Life Sciences (HUST) for instrument support.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
12274_2021_3525_MOESM1_ESM.pdf
Programmed albumin nanoparticles regulate immunosuppressive pivot to potentiate checkpoint blockade cancer immunotherapy
Rights and permissions
About this article
Cite this article
Feng, L., Yang, L., Li, L. et al. Programmed albumin nanoparticles regulate immunosuppressive pivot to potentiate checkpoint blockade cancer immunotherapy. Nano Res. 15, 593–602 (2022). https://doi.org/10.1007/s12274-021-3525-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-021-3525-6