Abstract
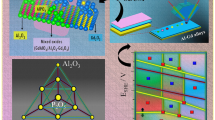
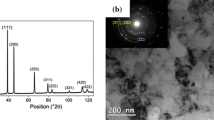
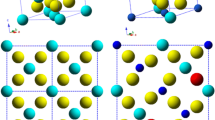
Multiple-principal element alloys hold great promise for multifunctional material discovery (e.g., for novel electrocatalysts based on complex solid solutions) in a virtually unlimited compositional space. Here, the phase constitution of the noble metal system Ag-Ir-Pd-Pt-Ru was investigated over a large compositional range in the quinary composition space and for different annealing temperatures from 600 to 900 °C using thin-film materials libraries. Composition-dependent X-ray diffraction mapping of the as-deposited thin-film materials library indicates different phases being present across the composition space (face-centered cubic (fcc), hexagonal close packed (hcp) and mixed fcc + hcp), which are strongly dependent on the Ru content. In general, low Ru contents promote the fcc phase, whereas high Ru contents favor the formation of an hcp solid-solution phase. Furthermore, a temperature-induced phase transformation study was carried out for a selected measurement area of fcc-Ag5Ir8Pd56Pt8Ru23. With increasing temperature, the initial fcc phase transforms to an intermediate C14-type Laves phase at 360 °C, and then to hcp when the temperature reaches 510 °C. The formation and disappearance of the hexagonal Laves phase, which covers a wide temperature range, plays a crucial role of bridging the fcc to hcp phase transition. The obtained composition, phase and temperature data are transformed into phase maps which could be used to guide theoretical studies and lay a basis for tuning the functional properties of these materials.

Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Yeh, J. W.; Chen, S. K.; Lin, S. J.; Gan, J. Y.; Chin, T. S.; Shun, T. T.; Tsau, C. H.; Chang, S. Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303.
Zhang, Y.; Zuo, T. T.; Tang, Z.; Gao, M. C.; Dahmen, K. A.; Liaw, P. K.; Lu, Z. P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 67, 1–93.
Tsai, M. H.; Yeh, J. W. High-entropy alloys: A critical review. Mater. Res. Lett. 2014, 2, 107–123.
Tsai, K. Y.; Tsai, M. H.; Yeh, J. W. Sluggish diffusion in Co-Cr-Fe-Mn-Ni high-entropy alloys. Acta Mater. 2013, 61, 4887–4897.
Murty, B. S.; Yeh, J. W.; Ranganathan, S.; Bhattacharjee, P. High-Entropy Alloys. Elsevier, 2019.
George, E. P.; Raabe, D.; Ritchie, R. O. High-entropy alloys. Nat. Rev. Mater. 2019, 4, 515–534.
Yeh, J. W. Recent progress in high-entropy alloys. Eur. J. Control. 2006, 31, 633–648.
Senkov, O. N.; Wilks, G. B.; Miracle, D. B.; Chuang, C. P.; Liaw, P. K. Refractory high-entropy alloys. Intermetallics 2010, 18, 1758–1765.
Hsu, Y. J.; Chiang, W. C.; Wu, J. K. Corrosion behavior of FeCoNiCrCu high-entropy alloys in 3.5% sodium chloride solution. Mater. Chem. Phys. 2005, 92, 112–117.
Kao, Y. F.; Chen, T. J.; Chen, S. K.; Yeh, J. W. Microstructure and mechanical property of as-cast, -homogenized, and -deformed AlxCoCrFeNi (0 ≤ x ≤ 2) high-entropy alloys. J. Alloys Compd. 2009, 488, 57–64.
Li, Z. Z.; Zhao, S. T.; Ritchie, R. O.; Meyers, M. A. Mechanical properties of high-entropy alloys with emphasis on face-centered cubic alloys. Prog. Mater. Sci. 2019, 102, 296–345.
Steurer, W. Single-phase high-entropy alloys —A critical update. Mater. Charact. 2020, 162, 110179.
Senkov, O. N.; Wilks, G. B.; Scott, J. M.; Miracle, D. B. Mechanical properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 refractory high entropy alloys. Intermetallics 2011, 19, 698–706.
Senkov, O. N.; Scott, J. M.; Senkova, S. V.; Miracle, D. B.; Woodward, C. F. Microstructure and room temperature properties of a high-entropy TaNbHfZrTi alloy. J. Alloys Compd. 2011, 509, 6043–6048.
Singh, P.; Smirnov, A. V.; Johnson, D. D. Ta-Nb-Mo-W refractory high-entropy alloys: Anomalous ordering behavior and its intriguing electronic origin. Phys. Rev. Mater. 2018, 2, 055004.
Youssef, K. M.; Zaddach, A. J.; Niu, C. N.; Irving, D. L.; Koch, C. C. A novel low-density, high-hardness, high-entropy alloy with close-packed single-phase nanocrystalline structures. Mater. Res. Lett. 2015, 3, 95–99.
Lužnik, J.; Koželj, P.; Vrtnik, S.; Jelen, A.; Jagličić, Z.; Meden, A.; Feuerbacher, M.; Dolinšek, J. Complex magnetism of Ho-Dy-Y-Gd-Tb hexagonal high-entropy alloy. Phys. Rev. B 2015, 92, 224201.
Soler, R.; Evirgen, A.; Yao, M.; Kirchlechner, C.; Stein, F.; Feuerbacher, M.; Raabe, D.; Dehm, G. Microstructural and mechanical characterization of an equiatomic YGdTbDyHo high entropy alloy with hexagonal close-packed structure. Acta Mater. 2018, 156, 86–96.
Pedersen, J. K.; Batchelor, T. A. A.; Bagger, A.; Rossmeisl, J. High-entropy alloys as catalysts for the CO2 and CO reduction reactions. ACS Catal. 2020, 10, 2169–2176.
Sohn, S.; Liu, Y. H.; Liu, J. B.; Gong, P.; Prades-Rodel, S.; Blatter, A.; Scanley, B. E.; Broadbridge, C. C.; Schroers, J. Noble metal high entropy alloys. Scr. Mater. 2017, 126, 29–32.
Yin, B. L.; Curtin, W. A. First-principles-based prediction of yield strength in the RhIrPdPtNiCu high-entropy alloy. npj Comput. Mater. 2019, 5, 14.
Yusenko, K. V.; Riva, S.; Carvalho, P. A.; Yusenko, M. V.; Arnaboldi, S.; Sukhikh, A. S.; Hanfland, M.; Gromilov, S. A. First hexagonal close packed high-entropy alloy with outstanding stability under extreme conditions and electrocatalytic activity for methanol oxidation. Scr. Mater. 2017, 138, 22–27.
Nellaiappan, S.; Katiyar, N. K.; Kumar, R.; Parui, A.; Malviya, K. D.; Pradeep, K. G.; Singh, A. K.; Sharma, S.; Tiwary, C. S.; Biswas, K. High-entropy alloys as catalysts for the CO2 and CO reduction reactions: Experimental realization. ACS Catal. 2020, 10, 3658–3663.
d’Acremont, Q.; Pernot, G.; Rampnoux, J. M.; Furlan, A.; Lacroix, D.; Ludwig, A.; Dilhaire, S. High-throughput heterodyne thermoreflectance: Application to thermal conductivity measurements of a Fe-Si-Ge thin film alloy library. Rev. Sci. Instrum. 2017, 88, 074902.
Khare, C.; Sliozberg, K.; Stepanovich, A.; Schuhmann, W.; Ludwig, A. Combinatorial synthesis and high-throughput characterization of structural and photoelectrochemical properties of Fe:WO3 nanostructured libraries. Nanotechnology 2017, 28, 185604.
Li, Z. M.; Ludwig, A.; Savan, A.; Springer, H.; Raabe, D. Combinatorial metallurgical synthesis and processing of high-entropy alloys. J. Mater. Res. 2018, 33, 3156–3169.
Lowry, M. S.; Hudson, W. R.; Pascal, R. A.; Bernhard, S. Accelerated luminophore discovery through combinatorial synthesis. J. Am. Chem. Soc. 2004, 126, 14129–14135.
Briceño, G.; Chang, H.; Sun, X. D.; Schultz, P. G.; Xiang, X. D. A class of cobalt oxide magnetoresistance materials discovered with combinatorial synthesis. Science 1995, 270, 273–275.
Wambach, M.; Nguyen, N.; Hamann, S.; Nishio, M.; Yagyu, S.; Chikyow, T.; Ludwig, A. Electrical and structural properties of the partial ternary thin-film system Ni-Si-B. ACS Comb. Sci. 2019, 21, 310–315.
Wang, X.; Rogalla, D.; Ludwig, A. Influences of W content on the phase transformation properties and the associated stress change in thin film substrate combinations studied by fabrication and characterization of thin film V1−xWxO2 materials libraries. ACS Comb. Sci. 2018, 20, 229–236.
Hanak, J. J. The “multiple-sample concept” in materials research: Synthesis, compositional analysis and testing of entire multicomponent systems. J. Mater. Sci. 1970, 5, 964–971.
Hanak, J. J.; Gittleman, J. I.; Pellicane, J. P.; Bozowski, S. The effect of grain size on the superconducting transition temperature of the transition metals. Phys. Lett. A 1969, 30, 201–202.
Böttcher, A.; Haase, G.; Thun, R. Strukturuntersuchung von Mehrstoffsystemen durch kinematische Elektronenbeugung. Z. Metallkde. 1955, 46, 386–400.
Schweizer, F.; Hindsgaul, O. Combinatorial synthesis of carbohydrates. Curr. Opin. Chem. Biol. 1999, 3, 291–298.
Li, Y. J.; Kostka, A.; Savan, A.; Ludwig, A. Atomic-scale investigation of fast oxidation kinetics of nanocrystalline CrMnFeCoNi thin films. J. Alloys Compd. 2018, 766, 1080–1085.
Ludwig, A. Discovery of new materials using combinatorial synthesis and high-throughput characterization of thin-film materials libraries combined with computational methods. npj Comput. Mater. 2019, 5, 70.
Naujoks, D.; Weiser, M.; Salomon, S.; Stein, H.; Virtanen, S.; Ludwig, A. Combinatorial study on phase formation and oxidation in the thin film superalloy subsystems Co-Al-Cr and Co-Al-Cr-W. ACS Comb. Sci. 2018, 20, 611–620.
Kumari, S.; Gutkowski, R.; Junqueira, J. R. C.; Kostka, A.; Hengge, K.; Scheu, C.; Schuhmann, W.; Ludwig, A. Combinatorial synthesis and high-throughput characterization of Fe-V-O thin-film materials libraries for solar water splitting. ACS Comb. Sci. 2018, 20, 544–553.
Batchelor, T. A. A.; Löffler, T.; Xiao, B.; Krysiak, O. A.; Strotkötter, V.; Pedersen, J. K.; Clausen, C. M.; Savan, A.; Li, Y. J.; Schuhmann, W. Complex-solid-solution electrocatalyst discovery by computational prediction and high-throughput experimentation. Angew. Chem., Int. Ed. 2020, 133, 7008–7013.
Liu, X. H.; Luo, J.; Zhu, J. Size effect on the crystal structure of silver nanowires. Nano Lett. 2006, 6, 408–412.
Guo, Q. X.; Zhao, Y. S.; Mao, W. L.; Wang, Z. W.; Xiong, Y. J.; Xia, Y. N. Cubic to tetragonal phase transformation in cold-compressed Pd nanocubes. Nano Lett. 2008, 8, 972–975.
Zhang, Q.; Kusada, K.; Wu, D. S.; Yamamoto, T.; Toriyama, T.; Matsumura, S.; Kawaguchi, S.; Kubota, Y.; Kitagawa, H. Selective control of fcc and hcp crystal structures in Au-Ru solid-solution alloy nanoparticles. Nat. Commun. 2018, 9, 510.
Rabadia, C. D.; Liu, Y. J.; Wang, L.; Sun, H.; Zhang, L. C. Laves phase precipitation in Ti-Zr-Fe-Cr alloys with high strength and large plasticity. Mater. Des. 2018, 154, 228–238.
Erdmann, B.; Keller, C. Actinide(lanthanide)-noble metal alloy phases, preparation and properties. J. Solid State Chem. 1973, 7, 40–48.
Compton, V. B.; Matthias, B. T. Laves phase compounds of rare earths and hafnium with noble metals. Acta Cryst. 1959, 12, 651–654.
Stein, F.; Palm, M.; Sauthoff, G. Structure and stability of Laves phases. Part I. Critical assessment of factors controlling Laves phase stability. Intermetallics 2004, 12, 713–720.
Scudino, S.; Donnadieu, P.; Surreddi, K. B.; Nikolowski, K.; Stoica, M.; Eckert, J. Microstructure and mechanical properties of Laves phase-reinforced Fe-Zr-Cr alloys. Intermetallics 2009, 17, 532–539.
Jiang, H.; Jiang, L.; Qiao, D. X.; Lu, Y. P.; Wang, T. M.; Cao, Z. Q.; Li, T. J. Effect of niobium on microstructure and properties of the CoCrFeNbxNi high entropy alloys. J. Mater. Sci. Technol. 2017, 33, 712–717.
Cheng, J. B.; Liang, X. B.; Xu, B. S. Effect of Nb addition on the structure and mechanical behaviors of CoCrCuFeNi high-entropy alloy coatings. Surf. Coat. Technol. 2014, 240, 184–190.
Zhou, Y. J.; Zhang, Y.; Wang, Y. L.; Chen, G. L. Solid solution alloys of Al Co Cr Fe Ni Tix with excellent room-temperature mechanical properties. Appl. Phys. Lett. 2007, 90, 181904.
Zhou, D.; Usher, B. F. Deviation of the AlGaAs lattice constant from Vegard’s law. J. Phys. D: Appl. Phys. 2001, 34, 1461.
Li, W.; Pessa, M.; Likonen, J. Lattice parameter in GaNAs epilayers on GaAs: Deviation from Vegard’s law. Appl. Phys. Lett. 2001, 78, 2864–2866.
Liu, L. G.; Bassett, W. A. Compression of Ag and phase transformation of NaCl. J. Appl. Phys. 1973, 44, 1475–1479.
Singh, H. P. Determination of thermal expansion of germanium, rhodium and iridium by X-rays. Acta Cryst 1968, A24, 469–471.
Bredig, G.; Allolio, R. Röntgenuntersuchungen an katalytisch wirkenden Metallen. Z. Phys. Chem. 1927, 126, 41–71.
Edwards, J. W.; Speiser, R.; Johnston, H. L. High temperature structure and thermal expansion of some metals as determined by X-ray diffraction data. I. Platinum, tantalum, niobium, and molybdenum. J. Appl. Phys. 1951, 22, 424–428.
Schroder, R. H.; Schmitz-Pranghe, N.; Kohlhaas, R. Experimentelle bestimmung der gitterparameter der platinmetalle im temperaturbereich -190 bis 1709 °C. Z. Metallkd. 1972, 63, 12–16.
Acknowledgements
ZGH is acknowledged for the use of its scientific infrastructure. This work was funded by Deutsche Forschungsgemeinschaft (DFG), projects LU1175/26-1 and LU1175/22-1.
Funding
Funding note Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
12274_2021_3516_MOESM1_ESM.pdf
Phase constitution of the noble metal thin-film complex solid solution system Ag-Ir-Pd-Pt-Ru in dependence of elemental compositions and annealing temperatures
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xiao, B., Wang, X., Savan, A. et al. Phase constitution of the noble metal thin-film complex solid solution system Ag-Ir-Pd-Pt-Ru in dependence of elemental compositions and annealing temperatures. Nano Res. 15, 4827–4836 (2022). https://doi.org/10.1007/s12274-021-3516-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-021-3516-7