Abstract
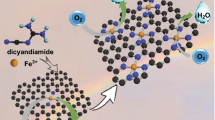
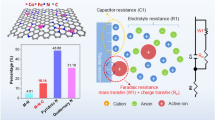
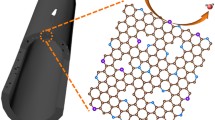
Despite acknowledgment of structural reconstruction of materials following oxygen evolution reaction (OER) reaction, the role of support during the reconstruction process has been ignored. Given this, we directly in situ transform the residual iron present in raw single-walled carbon nanotubes (SWCNT) into Fe2O3 and thus build Fe2O3-CNT as the model system. Intriguingly, an anomalous self-optimization occurred on SWCNT and the derived components show satisfactory electrochemical performance. Soft X-ray absorption spectroscopy (sXAS) analysis and theory calculation correspondingly indicate that self-optimization yields stronger interaction between SWCNT and Fe2O3 nanoparticles, where the electrons migrate from Fe2O3 to optimized SWCNT. Such polarization will generate a positive charge center and thus boost the OER activity. This finding directly observes the self-optimization of support effect, providing a new perspective for OER and related electrochemical reactions.

Similar content being viewed by others
References
Suen, N. T.; Hung, S. F.; Quan, Q.; Zhang, N.; Xu, Y. J.; Chen, H. M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365.
Song, F.; Bai, L. C.; Moysiadou, A.; Lee, S.; Hu, C.; Liardet, L.; Hu, X. L. Transition metal oxides as electrocatalysts for the oxygen evolution reaction in alkaline solutions: An application-inspired renaissance. J. Am. Chem. Soc. 2018, 140, 7748–7759.
Liu, X.; Wang, L.; Yu, P.; Tian, C. G.; Sun, F. F.; Ma, J. Y.; Li, W.; Fu, H. G. A stable bifunctional catalyst for rechargeable zinc-air batteries: Iron-cobalt nanoparticles embedded in a nitrogen-doped 3D carbon matrix. Angew. Chem., Int. Ed. 2018, 57, 16166–16170.
Dresp, S.; Luo, F.; Schmack, R.; Kühl, S.; Gliech, M.; Strasser, P. An efficient bifunctional two-component catalyst for oxygen reduction and oxygen evolution in reversible fuel cells, electrolyzers and rechargeable air electrodes. Energy Environ. Sci. 2016, 9, 2020–2024.
Zhu, Y. P.; Guo, C. X.; Zheng, Y.; Qiao, S. Z. Surface and interface engineering of noble-metal-free electrocatalysts for efficient energy conversion processes. Acc. Chem. Res. 2017, 50, 915–923.
Cao, D. F.; Liu, D. B.; Chen, S. M.; Moses, O. A.; Chen, X. J.; Xu, W. J.; Wu, C. Q.; Zheng, L. R.; Chu, S. Q.; Jiang, H. L. et al. Operando X-ray spectroscopy visualizing the chameleon-like structural reconstruction on an oxygen evolution electrocatalyst. Energy Environ. Sci. 2021, DOI: https://doi.org/10.1039/D0EE02276D.
Jiang, H. L.; He, Q.; Zhang, Y. K.; Song, L. Structural self-reconstruction of catalysts in electrocatalysis. Acc. Chem. Res. 2018, 57, 2968–2977.
Nam, D. H.; Bushuyev, O. S.; Li, J.; De Luna, P.; Seifitokaldani, A.; Dinh, C. T.; De Arquer, F. P. G.; Wang, Y. H.; Liang, Z. Q.; Proppe, A. H. et al. Metal-organic frameworks mediate Cu coordination for selective CO2 electroreduction. J. Am. Chem. Soc. 2018, 140, 11378–11386.
Sato, Y.; Kowalski, D.; Aoki, Y.; Habazaki, H. Long-term durability of platelet-type carbon nanofibers for OER and ORR in highly alkaline media. Appl. Catal. A Gen. 2020, 597, 117555.
Singh, H.; Zhuang, S. Q.; Ingis, B.; Nunna, B. B.; Lee, E. S. Carbon-based catalysts for oxygen reduction reaction: A review on degradation mechanisms. Carbon 2019, 151, 160–174.
Ross, P. N.; Sokol, H. The corrosion of carbon-black anodes in alkaline electrolyte: I. Acetylene black and the effect of cobalt catalyzation. J. Electrochem. Soc. 1984, 131, 1742–1750.
Pérez-Rodríguez, S.; Sebastián, D.; Lazáro, M. J. Insights on the electrochemical oxidation of ordered mesoporous carbons. J. Electrochem. Soc. 2020, 167, 024511.
Wang, H. F.; Chen, R. X.; Feng, J. Y.; Qiao, M.; Doszczeczko, S.; Zhang, Q.; Jorge, A. B.; Titirici, M. M. Freestanding non-precious metal electrocatalysts for oxygen evolution and reduction reactions. ChemelEctroChem 2018, 5, 1786–1804.
Filimonenkov, I. S.; Bouillet, C.; Kéranguéven, G.; Simonov, P. A.; Tsirlina, G. A.; Savinova, E. R. Carbon materials as additives to the OER catalysts: RRDE study of carbon corrosion at high anodic potentials. Electrochim. Acta 2019, 321, 134657.
Yang, J. C.; Park, S.; Choi, K. Y.; Park, H. S.; Cho, Y. G.; Ko, H.; Song, H. K. Activity-durability coincidence of oxygen evolution reaction in the presence of carbon corrosion: Case study of MnCo2O4 spinel with carbon black. ACS Sustainable Chem. Eng. 2018, 6, 9566–9571.
Jang, S. E.; Kim, H. Effect of water electrolysis catalysts on carbon corrosion in polymer electrolyte membrane fuel cells. J. Am. Chem. Soc. 2010, 132, 14700–14701.
Yi, Y. M.; Tornow, J.; Willinger, E.; Willinger, M. G.; Ranjan, C.; Schlögl, R. Electrochemical degradation of multiwall carbon nanotubes at high anodic potential for oxygen evolution in acidic media. ChemElectroChem 2015, 2, 1929–1937.
Liang, Y. Y.; Li, Y. G.; Wang, H. L.; Dai, H. J. Strongly coupled inorganic/nanocarbon hybrid materials for advanced electrocatalysis. J. Am. Chem. Soc. 2013, 135, 2013–2036.
Liang, Y. Y.; Wang, H. L.; Diao, P.; Chang, W.; Hong, G. S.; Li, Y. G.; Gong, M.; Xie, L. M.; Zhou, J. G.; Wang, J. et al. Oxygen reduction electrocatalyst based on strongly coupled cobalt oxide nanocrystals and carbon nanotubes. J. Am. Chem. Soc. 2012, 134, 15849–15857.
Wu, H.; Yang, T.; Du, Y. H.; Shen, L.; Ho, G. W. Identification of facet-governing reactivity in hematite for oxygen evolution. Adv. Mater. 2018, 30, 1804341.
McIntyre, N. S.; Zetaruk, D. G. X-ray photoelectron spectroscopic studies of iron oxides. Anal. Chem. 1977, 49, 1521–1529.
Allen, G. C.; Curtis, M. T.; Hooper, A. J.; Tucker, P. M. X-ray photoelectron spectroscopy of iron-oxygen systems. J. Chem. Soc. Dalton Trans. 1974, 1525–1530.
Sun, S. N.; Li, H. Y.; Xu, Z. J. Impact of surface area in evaluation of catalyst activity. Joule 2018, 2, 1024–1027.
Yang, X. L.; Li, H. N.; Lu, A. Y.; Min, S. X.; Idriss, Z.; Hedhili, M. N.; Huang, K. W.; Idriss, H.; Li, L. J. Highly acid-durable carbon coated Co3O4 nanoarrays as efficient oxygen evolution electrocatalysts. Nano Energy 2016, 25, 42–50.
Shao, Y. Y.; Dodelet, J. P.; Wu, G.; Zelenay, P. PGM-Free cathode catalysts for PEM fuel cells: A mini-review on stability challenges. Adv. Mater. 2019, 31, 1807615.
Kuznetsova, A.; Popova, I.; Yates, J. T., Jr.; Bronikowski, M. J.; Huffman, C. B.; Liu, J.; Smalley, R. E.; Hwu, H. H.; Chen, J. G. Oxygen-containing functional groups on single-wall carbon nanotubes: NEXAFS and vibrational spectroscopic studies. J. Am. Chem. Soc. 2001, 123, 10699–10704.
Feng, X. F.; Song, M. K.; Stolte, W. C.; Gardenghi, D.; Zhang, D.; Sun, X. H.; Zhu, J. F.; Cairns, E. J.; Guo, J. H. Understanding the degradation mechanism of rechargeable lithium/sulfur cells: A comprehensive study of the sulfur-graphene oxide cathode after discharge-charge cycling. Phys. Chem. Chem. Phys. 2014, 16, 16931–16940.
Gong, M.; Li, Y. G.; Wang, H. L.; Liang, Y. Y.; Wu, J. Z.; Zhou, J. G.; Wang, J.; Regier, T.; Wei, F.; Dai, H. J. An advanced Ni-Fe layered double hydroxide electrocatalyst for water oxidation. J. Am. Chem. Soc. 2013, 135, 8452–8455.
Liang, Y. Y.; Wang, H. L.; Zhou, J. G.; Li, Y. G.; Wang, J.; Regier, T.; Dai, H. J. Covalent hybrid of spinel manganese-cobalt oxide and graphene as advanced oxygen reduction electrocatalysts. J. Am. Chem. Soc. 2012, 134, 3517–3523.
Wu, J.; Xue, Y.; Yan, X.; Yan, W. S.; Cheng, Q. M.; Xie, Y. Co3O4 nanocrystals on single-walled carbon nanotubes as a highly efficient oxygen-evolving catalyst. Nano Res. 2012, 5, 521–530.
Augustsson, A.; Herstedt, M.; Guo, J. H.; Edström, K.; Zhuang, G. V.; Ross, P. N. Jr.; Rubensson, J. E.; Nordgren, J. Solid electrolyte interphase on graphite Li-ion battery anodes studied by soft X-ray spectroscopy. Phys. Chem. Chem. Phys. 2004, 6, 4185–4189.
He, K.; Tsega, T. T.; Liu, X.; Zai, J. T.; Li, X. H.; Liu, X. J.; Li, W. H.; Ali, N.; Qian, X. F. Utilizing the space-charge region of the FeNi-LDH/CoP p-n junction to promote performance in oxygen evolution electrocatalysis. Angew. Chem., Int. Ed. 2019, 58, 11903–11909.
Lin, Y. X.; Yang, L.; Zhang, Y. K.; Jiang, H. L.; Xiao, Z. J.; Wu, C. Q.; Zhang, G. B.; Jiang, J.; Song, L. Defective Carbon-CoP nanoparticles hybrids with interfacial charges polarization for efficient bifunctional oxygen electrocatalysis. Adv. Energy Mater. 2018, 8, 1703623.
Song, L.; Ci, L.; Lv, L.; Zhou, Z.; Yan, X.; Liu, D.; Yuan, H.; Gao, Y.; Wang, J.; Liu, L. et al. Direct synthesis of a macroscale single-walled carbon nanotube non-woven material. Adv. Mater. 2004, 16, 1529–1534.
Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Rad.2005, 12, 537–541.
Cao, D. F.; Ye, K.; Moses, O. A.; Xu, W. J.; Liu, D. B.; Song, P.; Wu, C. Q.; Wang, C. D.; Ding, S. Q.; Chen, S. M. et al. Engineering the in-plane structure of metallic phase molybdenum disulfide via Co and O dopants toward efficient alkaline hydrogen evolution. ACS Nano 2019, 13, 11733–11740.
Cui, B.; Lin, H.; Li, J. B.; Li, X.; Yang, J.; Tao, J. Core-ring structured NiCo2O4 nanoplatelets: Synthesis, characterization, and electrocatalytic applications. Adv. Funct. Mater. 2008, 18, 1440–1447.
Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561.
Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in Germanium. Phys. Rev. B 1994, 49, 14251–14269.
Perdew, J. P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868.
Dudarev, S. L.; Botton, G. A.; Savrasov, S. Y.; Humphreys, C. J.; Sutton, A. P. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA + U study. Phys. Rev. B 1998, 57, 1505–1509.
Pozun, Z. D.; Henkelman, G. Hybrid density functional theory band structure engineering in hematite. J. Chem. Phys. 2011, 134, 224706.
Adelstein, N.; Neaton, J. B.; Asta, M.; De Jonghe, L. C. Density functional theory based calculation of small-polaron mobility in hematite. Phys. Rev. B 2014, 89, 245115.
Acknowledgements
This work was financially supported in part by the National Key R&D Program of China (Nos. 2017YFA0303500 and 2020YFA0405800), National Natural Science Foundation of China (NSFC) (Nos. U1932201, U2032113, and 22075264), CAS Collaborative Innovation Program of Hefei Science Center (Nos. 2019HSC-CIP002 and 2020HSC-CIP002), the USTC Start-Up Fund, and CAS Interdisciplinary Innovation Team. L. S. acknowledges the support from Key Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), Nankai University (111 projects, B12015). We thank the Beijing Synchrotron Radiation Facility (1W1B, BSRF), the Hefei Synchrotron Radiation Facility (MCD-A and MCD-B Soochow Beamline for Energy Materials at NSRL), and the USTC Center for Micro and Nanoscale Research and Fabrication for help in characterizations.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Xu, W., Cao, D., Moses, O.A. et al. Probing self-optimization of carbon support in oxygen evolution reaction. Nano Res. 14, 4534–4540 (2021). https://doi.org/10.1007/s12274-021-3368-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-021-3368-1