Abstract
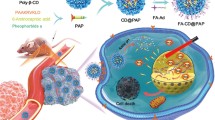
Photodynamic therapy (PDT) is a promising strategy for tumor treatment. Still, its therapeutic efficacy is compromised by the unsatisfactory cytotoxicity to specific subcellular organelles and insidious tumor microenvironment properties like hypoxia and high glutathione levels. Here, we fabricated a novel nanoenzyme that derived from metal-organic framework (MOF) with intrinsic catalase-like activities to decompose H2O2 to O2 and simultaneous glutathione consumption for enhancing PDT efficacy. The obtained Mn3O4 nanoparticle shows a larger pore size and surface area compared to native MOF particles, which can be used to load high dose photosensitizer. When decorated with AS1411 aptamer and polyethylene glycol (PEG), the obtained Mn3O4-PEG@C&A particle exhibits excellent stability and cell nucleus targeting ability. Remarkably, Mn3O4-PEG@C&A particle inhibited the tumor growth in the mouse model with high efficacy without any biotoxicity. This is the first report that applied MOF-derived nanoparticle to nucleus-targeted PDT. It may provide a new approach for designing functional nanoenzyme to subcellular organelles-targeted tumor modulation.

Similar content being viewed by others
References
Hanahan, D.; Weinberg, R. A. Hallmarks of cancer: The next generation. Cell2011, 144, 646–674.
Vander Heiden, M. G.; Cantley, L. C.; Thompson, C. B. Understanding the Warburg Effect: The metabolic requirements of cell proliferation. Science2009, 324, 1029–1033.
DeBerardinis, R. J.; Lum, J. J.; Hatzivassiliou, G.; Thompson, C. B. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab.2008, 7, 11–20.
Whiteside, T. L. The tumor microenvironment and its role in promoting tumor growth. Oncogene2008, 27, 5904–5912.
Cheng, Q.; Yu, W. Y.; Ye, J. J.; Liu, M. D.; Liu, W. L.; Zhang, C.; Feng, J.; Zhang, X. Z. Nanotherapeutics interfere with cellular redox homeostasis for highly improved photodynamic therapy. Biomaterials2019, 224, 119500.
Catalano, V.; Turdo, A.; Di Franco, S.; Dieli, F.; Todaro, M.; Stassi, G. Tumor and its microenvironment: A synergistic interplay. Semin. Cancer Biol.2013, 23, 522–532.
Fan, W. P.; Huang, P.; Chen, X. Y. Overcoming the Achilles’ heel of photodynamic therapy. Chem. Soc. Rev.2016, 45, 6488–6519.
Sahu, A.; Kwon, I.; Tae, G. Improving cancer therapy through the nanomaterials-assisted alleviation of hypoxia. Biomaterials2020, 228, 119578.
Vaupel, P. The role of hypoxia-induced factors in tumor progression. Oncologist2004, 9, 10–17.
Wang, L. Y.; Huo, M. F.; Chen, Y.; Shi, J. L. Tumor microenvironment-enabled nanotherapy. Adv. Healthcare Mater.2018, 7, 1701156.
Gong, F.; Cheng, L.; Yang, N. L.; Betzer, O.; Feng, L. Z.; Zhou, Q.; Li, Y. G.; Chen, R. H.; Popovtzer, Liu, Z. Ultrasmall oxygen-deficient bimetallic oxide MnWOX nanoparticles for depletion of endogenous GSH and enhanced sonodynamic cancer therapy. Adv. Mater.2019, 31, 1900730.
Cook, J. A.; Gius, D.; Wink, D. A.; Krishna, M. C.; Russo, A.; Mitchell, J. B. Oxidative stress, redox, and the tumor microenvironment. Semin. Radiat. Oncol.2004, 14, 259–266.
Dai, Y. L.; Xu, C.; Sun, X. L.; Chen, X. Y. Nanoparticle design strategies for enhanced anticancer therapy by exploiting the tumour microenvironment. Chem. Soc. Rev.2017, 46, 3830–3852.
Lu, Y.; Aimetti, A. A.; Langer, R.; Gu, Z. Bioresponsive materials. Nat. Rev. Mater.2017, 2, 16075.
Sun, W. J.; Hu, Q. Y.; Ji, W. Y.; Wright, G; Gu, Z. Leveraging physiology for precision drug delivery. Physiol. Rev.2017, 97, 189–225.
Cheng, Y. H.; Cheng, H.; Jiang, C. X.; Qiu, X. F.; Wang, K. K.; Huan, W.; Yuan, A. H.; Wu, J. H.; Hu, Y. Q. Perfluorocarbon nano-particles enhance reactive oxygen levels and tumour growth inhibition in photodynamic therapy. Nat. Commun.2015, 6, 8785.
Kim, J.; Cho, H. R.; Jeon, H.; Kim, D.; Song, C.; Lee, N.; Choi, S. H.; Hyeon, T. Continuous O2-evolving MnFe2O4 nanoparticle-anchored mesoporous silica nanoparticles for efficient photodynamic therapy in hypoxic cancer. J. Am. Chem. Soc.2017, 139, 10992–10995.
Fan, K. L.; Xi, J. Q.; Fan, L.; Wang, P. X.; Zhu, C. H.; Tang, Y.; Xu, X. D.; Liang, M. M.; Jiang, B.; Yan, X. Y. et al. In vivo guiding nitrogen-doped carbon nanozyme for tumor catalytic therapy. Nat. Commun.2018, 9, 1440.
Wei, J. P.; Li, J. C.; Sun, D.; Li, Q.; Ma, J. Y.; Chen, X. L.; Zhu, X.; Zheng, N. F. A novel theranostic nanoplatform based on Pd@Pt-PEG-Ce6 for enhanced photodynamic therapy by modulating tumor hypoxia microenvironment. Adv. Funct. Mater.2018, 28, 1706310.
Yang, Z. L.; Tian, W.; Wang, Q.; Zhao, Y.; Zhang, Y. L.; Tian, Y.; Tang. Y. X.; Wang, S. J.; Liu, Y.; Ni, Q. Q. et al. Oxygen-evolving mesoporous organosilica coated prussian blue nanoplatform for highly efficient photodynamic therapy of tumors. Adv. Sci.2018, 5, 1700847.
Huang, C. C.; Chia, W. T.; Chung, M. F.; Lin, K. J.; Hsiao, C. W.; Jin, C.; Lim, W. H.; Chen, C. C. Sung, H. W. An implantable depot that can generate oxygen in situ for overcoming hypoxia-induced resistance to anticancer drugs in chemotherapy. J. Am. Chem. Soc.2016, 138, 5222–5225.
Chen, H. C.; Tian, J. W.; He, W. J.; Guo, Z. J. H2O2-activatable and O2-evolving nanoparticles for highly efficient and selective photodynamic therapy against hypoxic tumor cells. J. Am. Chem. Soc.2015, 137, 1539–1547.
Zhang, X.; Xi, Z. Q.; Machuki, J. O.; Luo, J. J.; Yang, D. Z.; Li, J. J.; Cai, W. B., Yang, Y.; Zhang, L. J. et al. Gold cube-in-cube based oxygen nanogenerator: A theranostic nanoplatform for modulating tumor microenvironment for precise chemo-phototherapy and multimodal imaging. ACS Nano2019, 13, 5306–5325.
Liu, J. J.; Chen, Q.; Zhu, W. W.; Yi, X.; Yang, Y.; Dong, Z. L.; Liu, Z. Nanoscale-coordination-polymer-shelled manganese dioxide composite nanoparticles: A multistage redox/pH/H2O2-responsive cancer theranostic nanoplatform. Adv. Funct. Mater.2017, 27, 1605926.
Zhang, Y.; Wang, F. M.; Liu, C. Q.; Wang, Z. Z.; Kang, L. H.; Huang, Y. Y.; Dong, K.; Ren, J. S.; Qu, X. G. Nanozyme decorated metal—organic frameworks for enhanced photodynamic therapy. ACS Nano2018, 12, 651–661.
Zheng, D. W.; Li, B.; Li, C. X.; Fan, J. X.; Lei, Q.; Li, C.; Xu, Z. S.; Zhang, X. Z. Carbon-dot-decorated carbon nitride nanoparticles for enhanced photodynamic therapy against hypoxic tumor via water splitting. ACS Nano2016, 10, 8715–8722.
He, Z. M.; Huang, X. L.; Wang, C.; Li, X. L.; Liu, Y. J.; Zhou, Z. J.; Wang, S.; Zhang, F. W.; Wang, Z. T.; Jacobson, O. et al. A catalase-like metal-organic framework nanohybrid for O2-evolving synergistic chemoradiotherapy. Angew. Chem., Int. Ed.2019, 58, 8752–8756.
Min, H.; Wang, J.; Qi, Y. Q.; Zhang, Y. L.; Han, X. X.; Xu, Y.; Xu, J. C.; Li. Y.; Chen, L.; Cheng, K. M. et al. Biomimetic metal—organic framework nanoparticles for cooperative combination of antiangiogenesis and photodynamic therapy for enhanced efficacy. Adv. Mater.2019, 31, 1808200.
Liu, J. T.; Liu, T. R.; Du, P.; Zhang, L.; Lei, J. P. Metal—organic framework (MOF) hybrid as a tandem catalyst for enhanced therapy against hypoxic tumor cells. Angew. Chem., Int. Ed.2019, 58, 7808–7812.
Lan, G. X.; Ni, K. Y.; Xu, Z. W.; Veroneau, S. S.; Song, Y.; Lin, W. B. Nanoscale metal—organic framework overcomes hypoxia for photodynamic therapy primed cancer immunotherapy. J. Am. Chem. Soc.2018, 140, 5670–5673.
Wu, J. J. X; Li, S. R.; Wei, H. Integrated nanozymes: Facile preparation and biomedical applications. Chem. Commun.2018, 54, 6520–6530.
Cai, X. C.; Xie, Z. X.; Ding, B. B.; Shao, S.; Liang, S.; Pang, M. L.; Lin, J. Monodispersed copper(I)-based nano metal—organic framework as a biodegradable drug carrier with enhanced photodynamic therapy efficacy. Adv. Sci.2019, 6, 1900848.
Simon-Yarza, T.; Mielcarek, A.; Couvreur, P.; Serre, C. Nanoparticles of metal-organic frameworks: On the road to in vivo efficacy in biomedicine. Adv. Mater.2018, 30, 1707365.
Ranji-Burachaloo, H.; Karimi, F.; Xie, K.; Fu, Q.; Gurr, P. A.; Dunstan, D. E.; Qiao, G. G. MOF-mediated destruction of cancer using the cell’s own hydrogen peroxide. ACS Appl. Mater. Interfaces2017, 9, 33599–33608.
Wang, D. D.; Wu, H. H.; Lim, W. Q.; Phua, S. Z. F.; Xu, P. P.; Chen, Q. W.; Guo, Z.; Zhao, Y. L. A mesoporous nanoenzyme derived from metal—organic frameworks with endogenous oxygen generation to alleviate tumor hypoxia for significantly enhanced photodynamic therapy. Adv. Mater.2019, 31, 1901893.
Dang, S.; Zhu, Q. L.; Xu, Q. Nanomaterials derived from metal—organic frameworks. Nat. Rev. Mater.2018, 3, 17075.
Zhang, F. R.; Liu, Y. H.; Lei, J. N.; Wang, S. H.; Ji, X. M.; Liu, H. Y.; Yang, Q. Metal—organic-framework-derived carbon nanostructures for site-specific dual-modality photothermal/photodynamic thrombus therapy. Adv. Sci.2019, 6, 1901378.
Pan, X. T.; Bai, L. X.; Wang, H.; Wu, Q. Y.; Wang, H. Y.; Liu, S.; Xu, B. L.; Shi, X. H.; Liu, H. Y. Metal—organic-framework-derived carbon nanostructure augmented sonodynamic cancer therapy. Adv. Mater.2018, 30, 1800180.
Lin, L. S.; Song, J. B.; Song, L.; Ke, K. M.; Liu, Y. J.; Zhou, Z. J.; Shen, Z. Y.; Li, J.; Yang, Z.; Tang, W. et al. Simultaneous fenton-like ion delivery and glutathione depletion by MnO2-Based nanoagent to enhance chemodynamic therapy. Angew. Chem., Int. Ed.2018, 130, 4996–5000.
Chen, W. H.; Luo, G. F.; Zhang, X. Z. Recent advances in subcellular targeted cancer therapy based on functional materials. Adv. Mater.2019, 31, 1802725.
Pan, L. M.; Liu, J. N.; Shi, J. L. Cancer cell nucleus-targeting nano-composites for advanced tumor therapeutics. Chem. Soc. Rev.2018, 47, 6930–6946.
Pan, L. M.; He, Q. J.; Liu, J. N.; Chen, Y.; Ma, M.; Zhang, L. L.; Shi, J. L. Nuclear-targeted drug delivery of TAT peptide-conjugated monodisperse mesoporous silica nanoparticles. J. Am. Chem. Soc.2012, 134, 5722–5725.
Liu, J. N.; Bu, W. B.; Pan, L. M.; Zhang, S. J.; Chen, F.; Zhou, L. P.; Zhao, K. L.; Peng, W. J.; Shi, J. L. Simultaneous nuclear imaging and intranuclear drug delivery by nuclear-targeted multifunctional upconversion nanoprobes. Biomaterials2012, 33, 7282–7290.
Duan, J. J.; Chen, S.; Dai, S.; Qiao, S. Z. Shape control of Mn3O4 nanoparticles on nitrogen-doped graphene for enhanced oxygen reduction activity. Adv. Funct. Mater.2014, 24, 2072–2078.
Ouyang, J.; Wang, L. Q.; Chen, W. S.; Zeng, K.; Han, Y. J.; Xu, Y.; Xu, Q. F.; Xu, Q.; Deng, L.; Liu, Y. N. Biomimetic nanothylakoids for efficient imaging-guided photodynamic therapy for cancer. Chem. Commun.2018, 54, 3468–3471.
Zhai, S. D.; Hu, X. L.; Hu, Y. J.; Wu, B. Y.; Xing, D. Visible light-induced crosslinking and physiological stabilization of diselenide-rich nanoparticles for redox-responsive drug release and combination chemotherapy. Biomaterials2017, 121, 41–54.
Wu, J. J. X.; Wang, X. Y.; Wang, Q.; Lou. Z. P.; Li, S. R.; Zhu, Y. Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev.2019, 48, 1004–1076.
Cheng, L.; Wang, C.; Feng, L. Z.; Yang, K.; Liu, Z. Functional nanomaterials for phototherapies of cancer. Chem. Rev.2014, 114, 10869–10939.
Acknowledgements
We gratefully acknowledge the financial support from National Natural Science Foundation of China (Nos. 21775049, 31700746, 31870856 and 31870854) and National Key R&D Program of China (Nos. 2017YFA0700403 and 2016YFF0100801) and China Postdoctoral Science Foundation funded project (Nos. 2018M630847 and 2018T110753).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
12274_2020_2746_MOESM1_ESM.pdf
Modulation of tumor microenvironment by metal-organic-framework-derived nanoenzyme for enhancing nucleus-targeted photodynamic therapy
Rights and permissions
About this article
Cite this article
Zeng, X., Yan, S., Chen, P. et al. Modulation of tumor microenvironment by metal-organic-framework-derived nanoenzyme for enhancing nucleus-targeted photodynamic therapy. Nano Res. 13, 1527–1535 (2020). https://doi.org/10.1007/s12274-020-2746-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-020-2746-4