Abstract
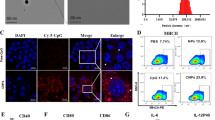
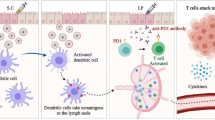
The maturation of dendritic cells (DCs) and infiltration effector T cells in tumor-draining lymph node (tdLN) and tumor tissue are crucial for immunotherapy. Despite constructive progresses have been made with anti-programmed death-1 (anti-PD1) checkpoint blockade for immunotherapy, the efficacy of PD1/PD-L1 therapy deserves to be improved. Here, we constructed a novel transfersomes based nanovaccine complexed microneedles to enhance anti-PD1 immunotherapy via transdermal immunization for skin tumor therapy. Transfersomes were functionalized with DCs targeting moiety αCD40, co-encapsulated with antigens and adjuvant poly I:C. Moreover, transdermal administration promoted accumulation in tumor-draining lymph nodes (tdLN), which could facilitate cellular uptake, activate DCs maturation and enhance Th1 immune responses. Using a mouse melanoma model, combined therapy of such nanovaccine complexed microneedles with pembrolizumab (αPD1) was able to enhance cytotoxic T lymphocytes activation, promote infiltration and reduce regulatory T cells frequency in tdLN and tumor tissues, which achieved reversion of the immunosuppressive microenvironment into immune activation. This study highlighted the potential of transfersomes based nanovaccines complexed microneedles as an attractive platform for tumor immunotherapy.

Similar content being viewed by others
References
Danhier, F.; Feron, O.; Préat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Releases2010, 148, 135–146.
Jeanbart, L.; Ballester, M.; de Titta, A.; Corthésy, P.; Romero, P.; Hubbell, J. A.; Swartz, M. A. Enhancing efficacy of anticancer vaccines by targeted delivery to tumor-draining lymph nodes. Cancer Immunol. Res.2014, 2, 436–447.
Rahimian, S.; Kleinovink, J. W.; Fransen, M. F.; Mezzanotte, L.; Gold, H.; Wisse, P.; Overkleeft, H.; Amidi, M.; Jiskoot, W.; Löwik, C. W. et al. Near-infrared labeled, ovalbumin loaded polymeric nanoparticles based on a hydrophilic polyester as model vaccine: In vivo tracking and evaluation of antigen-specific CD8+ T cell immune response. Biomaterials2015, 37, 469–477.
Ye, Y. Q.; Wang, J. Q.; Hu, Q. Y.; Hochu, G M.; Xin, H. L.; Wang, C.; Gu, Z. Synergistic transcutaneous immunotherapy enhances antitumor immune responses through delivery of checkpoint inhibitors. ACS Nano2016, 10, 8956–8963.
Sharma, P.; Allison, J. P. Immune checkpoint targeting in cancer therapy: Toward combination strategies with curative potential. Cell2015, 161, 205–214.
Yang, Y. P. Cancer immunotherapy: Harnessing the immune system to battle cancer. J. Clin. Invest.2015, 125, 3335–3337.
Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J. J.; Cowey, C. L.; Lao, C. D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P. et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. ngl. J. Med.2015, 373, 23–34.
Wang, C.; Ye, Y. Q.; Hochu, G. M.; Sadeghifa, H.; Gu, Z. Enhanced cancer immunotherapy by microneedle patch-assisted delivery of Anti-PD1 antibody. Nano Lett.2016, 16, 2334–2340.
Chandrasekaran, S.; King, M. Microenvironment of tumor-draining lymph nodes: Opportunities for liposome-based targeted therapy. Int. J. Mol. Sci.2014, 15, 20209–20239.
Fransen, M. F.; Arens, R.; Melief, C. J. M. Local targets for immune therapy to cancer: Tumor draining lymph nodes and tumor microenvironment. Int. J. Cancer2013, 132, 1971–1976.
de Wilt, J. H. W.; van Akkooi, A. C. J.; Verhoef, C.; Eggermont, A. M. M. Detection of melanoma micrometastases in sentinel nodes-The cons. Surg. Oncol.2008, 17, 175–181.
Tang, H. D.; Qiao, J.; Fu, Y. X. Immunotherapy and tumor microenvironment. Cancer Lett.2016, 370, 85–90.
Thomas, S. N.; Vokali, E.; Lund, A. W.; Hubbell, J. A.; Swartz, M. A. Targeting the tumor-draining lymph node with adjuvanted nano-particles reshapes the anti-tumor immune response. Biomaterials2014, 35, 814–824.
Malissen, B.; Tamoutounour, S.; Henri, S. The origins and functions of dendritic cells and macrophages in the skin. Nat. Rev. Immunol.2014, 14, 417–428.
Tacken, P. J.; Zeelenberg, I. S.; Cruz, L. J.; van Hout-Kuijer, M. A.; van de Glind, G.; Fokkink, R. G.; Lambeck, A. J. A.; Figdor, C. G. Targeted delivery of TLR ligands to human and mouse dendritic cells strongly enhances adjuvanticity. Blood2011, 118, 6836–6844.
Ye, Y. Q.; Wang, C.; Zhang, X. D.; Hu, Q. Y.; Zhang, Y. Q.; Liu, Q.; Wen, D.; Milligan, J.; Bellotti, A.; Huang, L. et al. A melanin-mediated cancer immunotherapy patch. Sci. Immunol.2017, 2, eaan5692.
Rahimian, S.; Fransen, M. F.; Kleinovink, J. W.; Amidi, M.; Ossendorp, F.; Hennink, W. E. Polymeric microparticles for sustained and local delivery of antiCD40 and antiCTLA-4 in immunotherapy of cancer. Biomaterials2015, 61, 33–40.
Rosalia, R. A.; Cruz, L. J.; van Duikeren, S.; Tromp, A. T.; Silva, A. L.; Jiskoot, W.; de Gruijl, T.; Löwik, C.; Oostendorp, J.; van der Burg, S. H. et al. CD40-targeted dendritic cell delivery of PLGA-nanoparticle vaccines induce potent anti-tumor responses. Biomaterials2015, 40, 88–97.
Schjetne, K. W.; Fredriksen, A. B.; Bogen, B. Delivery of antigen to CD40 induces protective immune responses against tumors. J. Immunol.2007, 178, 4169–4176.
Takemura, R.; Takaki, H.; Okada, S.; Shime, H.; Akazawa, T.; Oshiumi, H.; Matsumoto, M.; Teshima, T.; Seya, T. PolyI:C-induced, TLR3/RIP3-dependent necroptosis backs up immune effector-mediated tumor elimination in vivo. Cancer Immunol. Res.2015, 3, 902–914.
Lau, W. H.; Zhu, X. G.; Ho, S. W. T.; Chang, S. C.; Ding, J. L. Combinatorial treatment with polyI:C and anti-IL6 enhances apoptosis and suppresses metastasis of lung cancer cells. Oncotarget2017, 8, 32884–32904.
Frank-Bertoncelj, M.; Pisetsky, D. S.; Kolling, C.; Michel, B. A.; Gay, R. E.; Jüngel, A.; Gay, S. TLR3 ligand poly(I:C) exerts distinct actions in synovial fibroblasts when delivered by extracellular vesicles. Front. Immunol.2018, 9, 28.
Kong, M.; Hou, L.; Wang, J.; Feng, C.; Liu, Y.; Cheng, X. J.; Chen, X. G. Enhanced transdermal lymphatic drug delivery of hyaluronic acid modified transfersomes for tumor metastasis therapy. Chem. Commun.2015, 51, 1453–1456.
Ma, G. J.; Wu, C. W. Microneedle, bio-microneedle and bio-inspired microneedle: Areview. J. Control. Release2017, 251, 11–23.
Hao, Y.; Li, W.; Zhou, X. L.; Yang, F.; Qian, Z. Y. Microneedles-based transdermal drug delivery systems: A review. J. Biomed. Nanotechnol.2017, 13, 1581–1597.
Chen, G. J.; Chen, Z. T.; Wen, D.; Wang, Z. J.; Li, H. J.; Zeng, Y.; Dotti, G.; Wirz, R. E.; Gu, Z. Transdermal cold atmospheric plasmamediated immune checkpoint blockade therapy. Proc. Natl. Acad. Sci. USA2020, 117, 3687–3692.
Ye, Y. Q.; Yu, J. C.; Wen, D.; Kahkoska, A. R.; Gu, Z. Polymeric microneedles for transdermal protein delivery. Adv. Drug Deliv. Rev.2018, 127, 106–118.
Yang, H. J.; Wu, X. J.; Zhou, Z. Z.; Chen, X. G.; Kong, M. Enhanced transdermal lymphatic delivery of doxorubicin via hyaluronic acid based transfersomes/microneedle complex for tumor metastasis therapy. Int. J. Biol. Macromol.2019, 125, 9–16.
Wu, X. J.; Li, Y.; Chen, X. G.; Zhou, Z. Z.; Pang, J. H.; Luo, X.; Kong, M. A surface charge dependent enhanced Th1 antigen-specific immune response in lymph nodes by transfersome-based nanovaccine-loaded dissolving microneedle-assisted transdermal immunization. J. Mater. Chem. B2019, 7, 4854–4866.
Kong, M.; Park, H. J. Stability investigation of hyaluronic acid based nanoemulsion and its potential as transdermal carrier. Carbohydr. Polym.2011, 83, 1303–1310.
Robert, C.; Schachter, J.; Long, G. V.; Arance, A.; Grob, J. J.; Mortier, L.; Daud, A.; Carlino, M. S.; Mcneil, C.; Lotem, M. et al. Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl. J. Med.2015, 372, 2521–2532.
Kong, M.; Chen, X. G.; Park, H. Design and investigation of nanoemulsified carrier based on amphiphile-modified hyaluronic acid. Carbohydr. Polym.2011, 83, 462–469.
Sun, G. H.; Feng, C.; Jiang, C. Q.; Zhang, T. T.; Bao, Z. X.; Zuo, Y. J.; Kong, M.; Cheng, X. J.; Liu, Y.; Chen, X. G. Thermo-responsive hydroxybutyl chitosan hydrogel as artery intervention embolic agent for hemorrhage control. Int. J. Biol. Macromol.2017, 105, 566–574.
Rao, S. B.; Sharma, C. P. Use of chitosan as a biomaterial: Studies on its safety and hemostatic potential. J. Biomed. Mater. Res.1997, 34, 21–28.
Joffre, O. P.; Segura, E.; Savina, A.; Amigorena, S. Cross-presentation by dendritic cells. Nat. Rev. Immunol.2012, 12, 557–569.
Higa, K.; Shimmura, S.; Shimazaki, J.; Tsubota, K. Hyaluronic acid-CD44 interaction mediates the adhesion of lymphocytes by amniotic membrane stroma. Cornea2005, 24, 206–212.
Kong, M.; Zuo, Y. J.; Wang, M.; Bai, X. Y.; Feng, C.; Chen, X. G. Simply constructed chitosan nanocarriers with precise spatiotemporal control for efficient intracellular drug delivery. Carbohydr. Polym.2017, 169, 341–350.
Nawwab Al-Deen, F. M.; Selomulya, C.; Kong, Y. Y.; Xiang, S. D.; Ma, C.; Coppel, R. L.; Plebanski, M. Design of magnetic polyplexes taken up efficiently by dendritic cell for enhanced DNA vaccine delivery. Gene Ther.2014, 21, 212–218.
Liu, L. X.; Cao, F. Q.; Liu, X. X.; Wang, H.; Zhang, C.; Sun, H. F.; Wang, C.; Leng, X. G.; Song, C. X.; Kong, D. L. et al. Hyaluronic acid-modified cationic lipid-PLGA hybrid nanoparticles as a nanovaccine induce robust humoral and cellular immune responses. ACSAppl. Mater. Interfaces2016, 8, 11969–11979.
Randolph, G. J.; Angeli, V.; Swartz, M. A. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat. Rev. Immunol.2005, 5, 617–628.
Habijanic, J.; Berovic, M.; Boh, B.; Plankl, M.; Wraber, B. Submerged cultivation of Ganoderma lucidum and the effects of its polysaccharides on the production of human cytokines TNF-α, IL-12, IFN-γ, IL-2, IL-4, IL-10 and IL-17. New Biotechnol.2015, 32, 85–95.
Kane, L. P.; Andres, P. G.; Howland, K. C.; Abbas, A. K.; Weiss, A. Akt provides the CD28 costimulatory signal for up-regulation of IL-2 and IFN-γ but not TH2 cytokines. Nat. Immunol.2001, 2, 37–44.
Hong, Y.; Manoharan, I.; Suryawanshi, A.; Majumdar, T.; Angus-Hill, M. L.; Koni, P. A.; Manicassamy, B.; Mellor, A. L.; Munn, D. H.; Manicassamy, S. β-catenin promotes regulatory T-cell responses in tumors by inducing vitamin A metabolism in dendritic cells. Cancer Res.2015, 75, 656–665.
Acknowledgements
work was supported by the National Natural Science Foundation of China (No. 31670972), and the Taishan Scholar Program, China.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
12274_2020_2737_MOESM1_ESM.pdf
Reverse immune suppressive microenvironment in tumor draining lymph nodes to enhance anti-PD1 immunotherapy via nanovaccine complexed microneedle
Rights and permissions
About this article
Cite this article
Zhou, Z., Pang, J., Wu, X. et al. Reverse immune suppressive microenvironment in tumor draining lymph nodes to enhance anti-PD1 immunotherapy via nanovaccine complexed microneedle. Nano Res. 13, 1509–1518 (2020). https://doi.org/10.1007/s12274-020-2737-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-020-2737-5