Abstract
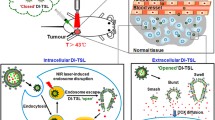
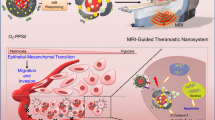
Real-time tracking drug release behavior is fundamentally important for avoiding adverse effects or unsuccessful treatment in personalize medical treatment. However, the development of a non-invasive drug reporting platform still remains challenging. Herein the design of a novel synthetic magnetic resonance imaging (MRI) agent for drug release tracking (SMART) is reported, which integrates photothermal core and paramagnetic ion/drug loading shell with a thermal valve in a hybrid structure. Through near-infrared (NIR)-II photothermal effect originating from inner Au-Cu9S5 nanohybrid core, burst release of drugs loaded in the mesoporous silica shell is achieved. The concomitant use of a phase change material not only prevents premature drug release, but also regulates heating effect, keeping local temperature below 45 °C, enabling synergistic chemotherapy and mild hyperthermia in vitro and in vivo. Furthermore, the drug release from SMART facilitates proton accessibility to the paramagnetic ions anchored inside mesopores channels, enhancing longitudinal T1 relaxation rate and displaying positive signal correlation to the amount of released drug, thus allowing non-invasive real-time monitoring of drug release event. The current study highlights the potential of designed MRI nanophores such as SMART for real-time and in-situ monitoring of drug delivery for precision theranostic applications.

Similar content being viewed by others
References
Li, C. A targeted approach to cancer imaging and therapy. Nat. Mater.2014, 13, 110–115.
Crawley, N.; Thompson, M.; Romaschin, A. Theranostics in the growing field of personalized medicine: An analytical chemistry perspective. Anal. Chem.2014, 86, 130–160.
El-Sawy, H. S.; Al-Abd, A. M.; Ahmed, T. A.; El-Say, K. M.; Torchilin, V. P. Stimuli-responsive nano-architecture drug-delivery systems to solid tumor micromilieu: Past, present, and future perspectives. ACS Nano2018, 12, 10636–10664.
Liu, Y.; Gong, C. S.; Dai, Y. L.; Yang, Z.; Yu, G. C.; Liu, Y. J.; Zhang, M. R.; Lin, L. S.; Tang, W.; Zhou, Z. J. In situ polymerization on nanoscale metal-organic frameworks for enhanced physiological stability and stimulus-responsive intracellular drug delivery. Biomaterials2019, 218, 119365.
Zheng, F. F.; Xiong, W. W.; Sun, S. S.; Zhang, P. H.; Zhu, J. J. Recent advances in drug release monitoring. Nanophotonics2019, 8, 391–413.
Moore, T.; Chen, H. Y.; Morrison, R.; Wang, F. L.; Anker, J. N.; Alexis, F. Nanotechnologies for noninvasive measurement of drug release. Mol. Pharmaceutics2014, 11, 24–39.
Lai, J. P.; Shah, B. P.; Garfunkel, E.; Lee, K. B. Versatile fluorescence resonance energy transfer-based mesoporous silica nanoparticles for real-time monitoring of drug release. ACS Nano2013, 7, 2741–2750.
Tian, L. M.; Gandra, N.; Singamaneni, S. Monitoring controlled release of payload from gold nanocages using surface enhanced Raman scattering. ACS Nano2013, 7, 4252–4260.
Wu, X. M.; Sun, X. R.; Guo, Z. Q.; Tang, J. B.; Shen, Y. Q.; James, T. D.; Tian, H.; Zhu, W. H. In vivo and in situ tracking cancer chemotherapy by highly photostable NIR fluorescent theranostic prodrug. J. Am. Chem. Soc.2014, 136, 3579–3588.
Wang, R.; Zhou, L.; Wang, W. X.; Li, X. M.; Zhang, F. In vivo gastrointestinal drug-release monitoring through second near-infrared window fluorescent bioimaging with orally delivered microcarriers. Nat. Commun.2017, 8, 14702.
Yu, X. J.; Yang, K.; Chen, X. Y.; Li, W. W. Black hollow silicon oxide nanoparticles as highly efficient photothermal agents in the second near-infrared window for in vivo cancer therapy. Biomaterials2017, 143, 120–129.
Liu, Z.; Ren, F.; Zhang, H.; Yuan, Q.; Jiang, Z. L.; Liu, H. H.; Sun, Q.; Li, Z. Boosting often overlooked long wavelength emissions of rare-earth nanoparticles for NIR-II fluorescence imaging of orthotopic glioblastoma. Biomaterials2019, 219, 119364.
Yang, Z.; Song, J. B.; Tang, W.; Fan, W. P.; Dai, Y. L.; Shen, Z. Y.; Lin, L. S.; Cheng, S. Y.; Liu, Y. J.; Niu, G. et al. Stimuli-responsive nanotheranostics for real-time monitoring drug release by photoacoustic imaging. Theranostics2019, 9, 526–536.
Bennett, K. M.; Jo, J. I.; Cabral, H.; Bakalova, R.; Aoki, I. MR imaging techniques for nano-pathophysiology and theranostics. Adv. Drug Delivery Rev.2014, 74, 75–94.
Janib, S. M.; Moses, A. S.; MacKay, J. A. Imaging and drug delivery using theranostic nanoparticles. Adv. Drug Delivery Rev.2010, 62, 1052–1063.
Viglianti, B. L.; Abraham, S. A.; Michelich, C. R.; Yarmolenko, P. S.; MacFall, J. R.; Bally, M. B.; Dewhirst, M. W. In vivo Monitoring of tissue pharmacokinetics of liposome/drug using MRI: Illustration of targeted delivery. Magn. Reson. Med.2004, 51, 1153–1162.
Ponce, A. M.; Viglianti, B. L.; Yu, D. H.; Yarmolenko, P. S.; Michelich, C. R.; Woo, J.; Bally, M. B.; Dewhirst, M. W. Magnetic resonance imaging of temperature-sensitive liposome release: Drug dose painting and antitumor effects. JNCI, J. Natl. Cancer Inst.2007, 99, 53–63.
Davies, G. L.; Kramberger, I.; Davis, J. J. Environmentally responsive MRI contrast agents. Chem. Commun.2013, 49, 9704–9721.
Van Duijnhoven, S. M. J.; Robillard, M. S.; Langereis, S.; Grüll, H. Bioresponsive probes for molecular imaging: Concepts and in vivo applications. Contrast Media Mol. Imaging2015, 10, 282–308.
Han, Y. X.; Zhou, X. X.; Qian, Y.; Hu, H. J.; Zhou, Z. X.; Liu, X. R.; Tang, J. B.; Shen, Y. Q. Hypoxia-targeting dendritic MRI contrast agent based on internally hydroxy dendrimer for tumor imaging. Biomaterials2019, 213, 119195.
Gao, Z. Y.; Hou, Y.; Zeng, J. F.; Chen, L.; Liu, C. Y.; Yang, W. S.; Gao, M. Y. Tumor microenvironment-triggered aggregation of antiphagocytosis 99mTc-Labeled Fe3O4 nanoprobes for enhanced tumor imaging in vivo. Adv. Mater.2017, 29, 1701095.
Zhao, Z. L.; Fan, H. H.; Zhou, G. F.; Bai, H. R.; Liang, H.; Wang, R. W.; Zhang, X. B.; Tan, W. H. Activatable fluorescence/MRI bimodal platform for tumor cell imaging via MnO2 nanosheet-aptamer nanoprobe. J. Am. Chem. Soc.2014, 136, 11220–11223.
Do, Q. N.; Ratnakar, J. S.; Kovács, Z.; Sherry, A. D. Redox- and hypoxia-responsive MRI contrast agents. ChemMedChem2014, 9, 1116–1129.
Fu, D. D.; Ding, X. G.; Wu, J.; Li, C. Y.; Wang, Q. B.; Jiang, J. Cationic polyelectrolyte mediated synthesis of MnO2-based core-shell structures as activatable MRI theranostic platform for tumor cell ablation. Part. Part. Syst. Charact.2018, 35, 1800078.
Kaittanis, C.; Shaffer, T. M.; Ogirala, A.; Santa, S.; Perez, J. M.; Chiosis, G.; Li, Y. M.; Josephson, L.; Grimm, J. Environment-responsive nanophores for therapy and treatment monitoring via molecular MRI quenching. Nat. Commun.2014, 5, 3384.
Zhao, Z. H.; Wang, X. M.; Zhang, Z. J.; Zhang, H.; Liu, H. Y.; Zhu, X. L.; Li, H.; Chi, X. Q.; Yin, Z. Y.; Gao, J. H. Real-time monitoring of arsenic trioxide release and delivery by activatable T1 imaging. ACS Nano2015, 9, 2749–2759.
Lu, J. X.; Sun, J. H.; Li, F. Y.; Wang, J.; Liu, J. N.; Kim, D.; Fan, C. H.; Hyeon, T.; Ling, D. S. Highly sensitive diagnosis of small hepatocellular carcinoma using pH-responsive iron oxide nanocluster assemblies. J. Am. Chem. Soc.2018, 140, 10071–10074.
Viger, M. L.; Sankaranarayanan, J.; de Gracia Lux, C.; Chan, M. N.; Almutairi, A. Collective activation of MRI agents via encapsulation and disease-triggered release. J. Am. Chem. Soc.2013, 135, 7847–7850.
Dong, Z. L.; Feng, L. Z.; Zhu, W. W.; Sun, X. Q.; Gao, M.; Zhao, H.; Chao, Y.; Liu, Z. CaCO3 nanoparticles as an ultra-sensitive tumor-pH-responsive nanoplatform enabling real-time drug release monitoring and cancer combination therapy. Biomaterials2016, 110, 60–70.
He, Z. M.; Zhang, P. H.; Xiao, Y.; Li, J. J.; Yang, F.; Liu, Y.; Zhang, J. R.; Zhu, J. J. Acid-degradable gadolinium-based nanoscale coordination polymer: A potential platform for targeted drug delivery and potential magnetic resonance imaging. Nano Res.2018, 11, 929–939.
Liu, J. N.; Bu, J. W.; Bu, W.; Zhang, S. J.; Pan, L. M.; Fan, W. P.; Chen, F.; Zhou, L. P.; Peng, W. J.; Zhao, K. L. et al. Real-time in vivo quantitative monitoring of drug release by dual-mode magnetic resonance and upconverted luminescence imaging. Angew. Chem., Int. Ed.2014, 53, 4551–4555.
Liang, C.; Xu, L. G.; Song, G. S.; Liu, Z. Emerging nanomedicine approaches fighting tumor metastasis: Animal models, metastasis-targeted drug delivery, phototherapy, and immunotherapy. Chem. Soc. Rev.2016, 45, 6250–6269.
Cheng, L.; Wang, C.; Feng, L. Z.; Yang, K.; Liu, Z. Functional nanomaterials for phototherapies of cancer. Chem. Rev.2014, 114, 10869–10939.
Zhong, D. N.; Zhao, J.; Li, Y. Y.; Qiao, Y.; Wei, Q. L.; He, J.; Xie, T. T.; Li, W. L.; Zhou, M. Laser-triggered aggregated cubic α-Fe2O3@Au nano-composites for magnetic resonance imaging and photothermal/enhanced radiation synergistic therapy. Biomaterials2019, 219, 119369.
Luo, D. D.; Carter, K. A.; Miranda, D.; Lovell, J. F. Chemophototherapy: An emerging treatment option for solid tumors. Adv. Sci.2017, 4, 1600106.
Zhao, Z. H.; Xu, K.; Fu, C.; Liu, H.; Lei, M.; Bao, J. F.; Fu, A. L.; Yu, Y.; Zhang, W. G. Interfacial engineered gadolinium oxide nanoparticles for magnetic resonance imaging guided microenvironment-mediated synergetic chemodynamic/photothermal therapy. Biomaterials2019, 119379.
Agarwal, A.; Mackey, M. A.; El-Sayed, M. A.; Bellamkonda, R. V. Remote triggered release of doxorubicin in tumors by synergistic application of thermosensitive liposomes and gold nanorods. ACS Nano2011, 5, 4919–4926.
Kong, G.; Braun, R. D.; Dewhirst, M. W. Characterization of the effect of hyperthermia on nanoparticle extravasation from tumor vasculature. Cancer Res.2001, 61, 3027–3032.
Hauck, T. S.; Jennings, T. L.; Yatsenko, T.; Kumaradas, J. C.; Chan, W. C. W. Enhancing the toxicity of cancer chemotherapeutics with gold nanorod hyperthermia. Adv. Mater.2008, 20, 3832–3838.
Melamed, J. R.; Edelstein, R. S.; Day, E. S. Elucidating the fundamental mechanisms of cell death triggered by photothermal therapy. ACS Nano2015, 9, 6–11.
Liu, Y. J.; Bhattarai, P.; Dai, Z. F.; Chen, X. Y. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev.2019, 48, 2053–2108.
Carrasco, E.; del Rosal, B.; Sanz-Rodríguez, F.; de la Fuente, Á. J.; Gonzalez, P. H.; Rocha, U.; Kumar, K. U.; Jacinto, C.; Solé, J. G.; Jaque, D. Intratumoral thermal reading during photo-thermal therapy by multifunctional fluorescent nanoparticles. Adv. Funct. Mater.2015, 25, 615–626.
Karan, N. S.; Keller, A. M.; Sampat, S.; Roslyak, O.; Arefin, A.; Hanson, C. J.; Casson, J. L.; Desireddy, A.; Ghosh, Y.; Piryatinski, A. et al. Plasmonic giant quantum dots: Hybrid nanostructures for truly simultaneous optical imaging, photothermal effect and thermometry. Chem. Sci.2015, 6, 2224–2236.
Yang, Y.; Zhu, W. J.; Dong, Z. L.; Chao, Y.; Xu, L.; Chen, M.; Liu, Z. 1D coordination polymer nanofibers for low-temperature photothermal therapy. Adv. Mater.2017, 29, 1703588.
Zhang, K.; Meng, X. D.; Cao, Y.; Yang, Z.; Dong, H. F.; Zhang, Y. D.; Lu, H. T.; Shi, Z. J.; Zhang, X. J. Metal-organic framework nanoshuttle for synergistic photodynamic and low-temperature photothermal therapy. Adv. Funct. Mater.2018, 28, 1804634.
Malzahn, K.; Ebert, S.; Schlegel, I.; Neudert, O.; Wagner, M.; Schütz, G.; Ide, A.; Roohi, F.; Münnemann, K.; Crespy, D. et al. Design and control of nanoconfinement to achieve magnetic resonance contrast agents with high relaxivity. Adv. Healthc. Mater.2016, 5, 567–574.
Ananta, J. S.; Godin, B.; Sethi, R.; Moriggi, L.; Liu, X. W.; Serda, R. E.; Krishnamurthy, R.; Muthupillai, R.; Bolskar, R. D.; Helm, L. et al. Geometrical confinement of gadolinium-based contrast agents in nanoporous particles enhances T1 Contrast. Nat. Nanotechnol.2010, 5, 815–821.
Ding, X. G.; Liow, C. H.; Zhang, M. X.; Huang, R. J.; Li, C. Y.; Shen, H.; Liu, M. Y.; Zou, Y.; Gao, N.; Zhang, Z. J. et al. Surface plasmon resonance enhanced light absorption and photothermal therapy in the second near-infrared window. J. Am. Chem. Soc.2014, 136, 15684–15693.
Moon, G. D.; Choi, S. W.; Cai, X.; Li, W. Y.; Cho, E. C.; Jeong, U.; Wang, L. V.; Xia, Y. N. A new theranostic system based on gold nanocages and phase-change materials with unique features for photoacoustic imaging and controlled release. J. Am. Chem. Soc.2011, 133, 4762–4765.
Cui, J. B.; Jiang, R.; Guo, C.; Bai, X. L.; Xu, S. Y.; Wang, L. Y. Fluorine grafted Cu7S4-Au heterodimers for multimodal imaging guided photothermal therapy with high penetration depth. J. Am. Chem. Soc.2018, 140, 5890–5894.
Liu, X.; Lee, C.; Law, W. C.; Zhu, D. W.; Liu, M. X.; Jeon, M.; Kim, J.; Prasad, P. N.; Kim, C.; Swihart, M. T. Au-Cu2−xSe heterodimer nanoparticles with broad localized surface plasmon resonance as contrast agents for deep tissue imaging. Nano Lett.2013, 13, 4333–4339.
Zhu, D. W.; Liu, M. X.; Liu, X.; Liu, Y.; Prasad, P. N.; Swihart, M. T. Au-Cu2−xSe heterogeneous nanocrystals for efficient photothermal heating for cancer therapy. J. Mater. Chem. B2017, 5, 4934–4942.
Zou, Y.; Sun, C.; Gong, W. B.; Yang, X. F.; Huang, X.; Yang, T.; Lu, W. B.; Jiang, J. Morphology-controlled synthesis of hybrid nanocrystals via a selenium-mediated strategy with ligand shielding effect: The case of dual plasmonic Au-Cu2−xSe. ACS Nano2017, 11, 3776–3785.
Zhu, H.; Wang, Y.; Chen, C.; Ma, M. R.; Zeng, J. F.; Li, S. Z.; Xia, Y. S.; Gao, M. Y. Monodisperse dual plasmonic Au@Cu2−xE (E= S, Se) core@shell supraparticles: Aqueous fabrication, multimodal imaging, and tumor therapy at in vivo level. ACS Nano2017, 11, 8273–8281.
Ji, M. W.; Xu, M.; Zhang, W.; Yang, Z. Z.; Huang, L.; Liu, J. J.; Zhang, Y.; Gu, L.; Yu, Y. X.; Hao, W. C. et al. Structurally well-defined Au@Cu2−xS core-shell nanocrystals for improved cancer treatment based on enhanced photothermal efficiency. Adv. Mater.2016, 28, 3094–3101.
Chang, Y.; Cheng, Y.; Feng, Y. L.; Jian, H.; Wang, L.; Ma, X. M.; Li, X.; Zhang, H. Y. Resonance energy transfer-promoted photothermal and photodynamic performance of gold-copper sulfide yolk-shell nanoparticles for chemophototherapy of cancer. Nano Lett.2018, 18, 886–897.
Wang, X. D.; Guo, L. R.; Zhang, S. H.; Chen, Y.; Chen, Y. T.; Zheng, B. B.; Sun, J. W.; Qian, Y. Y.; Chen, Y. X.; Yan, B. F. et al. Copper sulfide facilitates hepatobiliary clearance of gold nanoparticles through the copper-transporting ATPase ATP7B. ACS Nano2019, 13, 5720–5730.
Liu, X. J.; Deng, G. Y.; Wang, Y. Y.; Wang, Q.; Gao, Z. F.; Sun, Y. G.; Zhang, W. L.; Lu, J.; Hu, J. Q. A novel and facile synthesis of porous SiO2-coated ultrasmall Se particles as a drug delivery nanoplatform for efficient synergistic treatment of cancer cells. Nanoscale2016, 8, 8536–8541.
Kim, T.; Momin, E.; Choi, J.; Yuan, K.; Zaidi, H.; Kim, J.; Park, M.; Lee, N.; McMahon, M. T.; Quinones-Hinojosa, A. et al. Mesoporous silica-coated hollow manganese oxide nanoparticles as positive T1 contrast agents for labeling and MRI tracking of adipose-derived mesenchymal stem cells. J. Am. Chem. Soc.2011, 133, 2955–2961.
Huang, C. C.; Tsai, C. Y.; Sheu, H. S.; Chuang, K. Y.; Su, C. H.; Jeng, U. S.; Cheng, F. Y.; Su, C. H.; Lei, H. Y.; Yeh, C. S. Enhancing transversal relaxation for magnetite nanoparticles in MR imaging using Gd3+-chelated mesoporous silica shells. ACS Nano2011, 5, 3905–3916.
Chen, Y.; Ding, X. G.; Zhang, Y.; Natalia, A.; Sun, X. C.; Wang, Z. G.; Shao, H. L. Design and synthesis of magnetic nanoparticles for biomedical diagnostics. Quant. Imaging Med. Surg.2018, 8, 957–970.
Gosselin, R. E.; Hodge, H. C.; Smith, R. P.; Gleason, M. N. Clinical Toxicology of Commercial Products: Acute Poisoning; 4th ed. Williams & Wilkins: Baltimore, 1976.
Alcohol C-14 myristic. Food Cosmet. Toxicol.1975, 13, 699–700.
Rizzo, W. B. Fatty aldehyde and fatty alcohol metabolism: Review and importance for epidermal structure and function. Biochim. Biophys. Acta, Mol. Cell Biol. Lipids2014, 1841, 377–389.
Aznar, E.; Mondragón, L.; Ros-Lis, J. V.; Sancenón, F.; Marcos, M. D.; Martínez-Máñez, R.; Soto, J.; Pérez-Payá E.; Amorós, P. Finely tuned temperature-controlled cargo release using paraffin-capped mesoporous silica nanoparticles. Angew. Chem., Int. Ed.2011, 50, 11172–11175.
Oró, E.; De Gracia, A.; Castell, A.; Farid, M. M.; Cabeza, L. F. Review on phase change materials (PCMs) for cold thermal energy storage applications. Appl. Energy2012, 99, 513–533.
Zalba, B.; Marín, J. M.; Cabeza, L. F.; Mehling, H. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications. Appl. Therm. Eng.2003, 23, 251–283.
Zhang, X.; Du, J. F.; Guo, Z.; Yu, J.; Gao, Q.; Yin, W. Y.; Zhu, S.; Gu, Z. J.; Zhao, Y. L. Efficient near infrared light triggered nitric oxide release nano-composites for sensitizing mild photothermal therapy. Adv. Sci.2019, 6, 1801122.
Chen, Q.; Hu, Q. Y.; Dukhovlinova, E.; Chen, G. J.; Ahn, S.; Wang, C.; Ogunnaike, E. A.; Ligler, F. S.; Dotti, G.; Gu, Z. Photothermal therapy promotes tumor infiltration and antitumor activity of CAR T cells. Adv. Mater.2019, 31, 1900192.
Acknowledgements
This work was funded by the National Natural Science Foundation of China (No. 21473243) and Six Talent Peaks Project in Jiangsu Province (No. SWYY-243).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
12274_2019_2540_MOESM1_ESM.pdf
All-in-one theranostic nanoplatform with controlled drug release and activated MRI tracking functions for synergistic NIR-II hyperthermia-chemotherapy of tumors
Rights and permissions
About this article
Cite this article
Ding, X., Zhao, H., Li, C. et al. All-in-one theranostic nanoplatform with controlled drug release and activated MRI tracking functions for synergistic NIR-II hyperthermia-chemotherapy of tumors. Nano Res. 12, 2971–2981 (2019). https://doi.org/10.1007/s12274-019-2540-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-019-2540-3