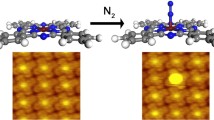
Abstract
Fundamental understanding of chemistry confined to nanospace remains a challenge since molecules encapsulated in confined microenvironments are difficult to be characterized. Here, we show that CO adsorption on Pt(111) confined under monolayer hexagonal boron nitride (h-BN) can be dynamically imaged using near ambient pressure scanning tunneling microscope (NAP-STM) and thanks to tunneling transparency of the top h-BN layer. The observed CO superstructures on Pt(111) in different CO atmospheres allow to derive surface coverages of CO adlayers, which are higher in the confined nanospace between h-BN and Pt(111) than those on the open Pt surface under the same conditions. Dynamic NAP-STM imaging data together with theoretical calculations confirm confinement-induced molecule enrichment effect within the 2D nanospace, which reveals new chemistry aroused by the confined nanoreactor.

Similar content being viewed by others
References
Petrosko, S. H.; Johnson, R.; White, H.; Mirkin, C. A. Nanoreactors: Small spaces, big implications in chemistry. J. Am. Chem. Soc. 2016, 138, 7443–7445.
Koblenz, T. S.; Wassenaar, J.; Reek, J. N. H. Reactivity within a confined self-assembled nanospace. Chem. Soc. Rev. 2008, 37, 247–262.
Fu, Q.; Bao, X. H. Surface chemistry and catalysis confined under two-dimensional materials. Chem. Soc. Rev. 2017, 46, 1842–1874.
Gounder, R.; Iglesia, E. The catalytic diversity of zeolites: confinement and solvation effects within voids of molecular dimensions. Chem. Commun. 2013, 49, 3491–3509.
Janda, A.; Vlaisavljevich, B.; Lin, L. C.; Smit, B.; Bell, A. T. Effects of zeolite structural confinement on adsorption thermodynamics and reaction kinetics for monomolecular cracking and dehydrogenation of n-butane. J. Am. Chem. Soc. 2016, 138, 4739–4756.
Sastre, G.; Corma, A. The confinement effect in zeolites. J. Mol. Catal. A Chem. 2009, 305, 3–7.
Miners, S. A.; Rance, G. A.; Khlobystov, A. N. Chemical reactions confined within carbon nanotubes. Chem. Soc. Rev. 2016, 45, 4727–4746.
Pan, X. L.; Bao, X. H. The effects of confinement inside carbon nanotubes on catalysis. ACC. Chem. Res. 2011, 44, 553–562.
Li, H. B.; Xiao, J. P.; Fu, Q.; Bao, X. H. Confined catalysis under twodimensional materials. Proc. Natl. Acad. Sci. USA 2017, 114, 5930–5934.
Doyle, A. D.; Montoya, J. H.; Vojvodic, A. Improving oxygen electrochemistry through nanoscopic confinement. ChemCatChem 2015, 7, 738–742.
Kovtyukhova, N. I.; Wang, Y. X.; Berkdemir, A.; Cruz-Silva, R.; Terrones, M.; Crespi, V. H.; Mallouk, T. E. Non-oxidative intercalation and exfoliation of graphite by Brønsted acids. Nat. Chem. 2014, 6, 957–963.
Yu, C. G.; He, J. Synergic catalytic effects in confined spaces. Chem. Commun. 2012, 48, 4933–4940.
Ferrighi, L.; Datteo, M.; Fazio, G.; Di Valentin, C. Catalysis under cover: Enhanced reactivity at the interface between (doped) graphene and anatase TiO2. J. Am. Chem. Soc. 2016, 138, 7365–7376.
Prieto, M. J.; Klemm, H. W.; Xiong, F.; Gottlob, D. M.; Menzel, D.; Schmidt, T.; Freund, H. J. Water formation under silica thin films: Real-time observation of a chemical reaction in a physically confined space. Angew. Chem., Int. Ed. 2018, 57, 8749–8753.
Yang, F.; Deng, D. H.; Pan, X. L.; Fu, Q.; Bao, X. H. Understanding nano effects in catalysis. Natl. Sci. Rev. 2015, 2, 183–201.
Xiao, J. P.; Pan, X. L.; Guo, S. J.; Ren, P. J.; Bao, X. H. Toward fundamentals of confined catalysis in carbon nanotubes. J. Am. Chem. Soc. 2015, 137, 477–482.
Deng, D. H.; Novoselov, K. S.; Fu, Q.; Zheng, N. F.; Tian, Z. Q.; Bao, X. H. Catalysis with two-dimensional materials and their heterostructures. Nat. Nanotechnol. 2016, 11, 218–230.
Feng, X. F.; Maier, S.; Salmeron, M. Water splits epitaxial graphene and intercalates. J. Am. Chem. Soc. 2012, 134, 5662–5668.
Yao, Y. X.; Fu, Q.; Zhang, Y. Y.; Weng, X. F.; Li, H.; Chen, M. S.; Jin, L.; Dong, A. Y.; Mu, R. T.; Jiang, P. et al. Graphene cover-promoted metalcatalyzed reactions. Proc. Natl. Acad. Sci. USA 2014, 111, 17023–17028.
Mu, R. T.; Fu, Q.; Jin, L.; Yu, L.; Fang, G. Z.; Tan, D. L.; Bao, X. H. Visualizing chemical reactions confined under graphene. Angew. Chem., Int. Ed. 2012, 51, 4856–4859.
Zhou, Y. N.; Chen, W.; Cui, P.; Zeng, J.; Lin, Z. N.; Kaxiras, E.; Zhang, Z. Y. Enhancing the hydrogen activation reactivity of nonprecious metal substrates via confined catalysis underneath graphene. Nano Lett. 2016, 16, 6058–6063.
Sutter, P.; Sadowski, J. T.; Sutter, E. A. Chemistry under cover: Tuning metal-graphene interaction by reactive intercalation. J. Am. Chem. Soc. 2010, 132, 8175–8179.
Jiao, F.; Li, J. J.; Pan, X. L.; Xiao, J. P.; Li, H. B.; Ma, H.; Wei, M. M.; Pan, Y.; Zhou, Z. Y.; Li, M. R. et al. Selective conversion of syngas to light olefins. Science 2016, 351, 1065–1068.
Ratnasamy, C.; Wagner, J. P. Water gas shift catalysis. Catal. Rev. 2009, 51, 325–440.
Ding, K. L.; Gulec, A.; Johnson, A. M.; Schweitzer, N. M.; Stucky, G. D.; Marks, L. D.; Stair, P. C. Identification of active sites in CO oxidation and water-gas shift over supported Pt catalysts. Science 2015, 350, 189–192.
Zhang, Y. H.; Weng, X. F.; Li, H.; Li, H. B.; Wei, M. M.; Xiao, J. P.; Liu, Z.; Chen, M. S.; Fu, Q.; Bao, X. H. Hexagonal boron nitride cover on Pt(111): A new route to tune molecule-metal interaction and metal-catalyzed reactions. Nano Lett. 2015, 15, 3616–3623.
Wei, M. M.; Fu, Q.; Yang, Y.; Wei, W.; Crumlin, E.; Bluhm, H.; Bao, X. H. Modulation of surface chemistry of Co on NI(111) by surface graphene and carbidic carbon. J. Phys. Chem. C 2015, 119, 13590–13597.
Nilsson, L.; Andersen, M.; Balog, R.; Lægsgaard, E.; Hofmann, P.; Besenbacher, F.; Hammer, B.; Stensgaard, I.; Hornekær, L. Graphene coatings: Probing the limits of the one atom thick protection layer. ACS Nano 2012, 6, 10258–10266.
Grånäs, E.; Andersen, M.; Arman, M. A.; Gerber, T.; Hammer, B.; Schnadt, J.; Andersen, J. N.; Michely, T.; Knudsen, J. CO intercalation of graphene on Ir(111) in the millibar regime. J. Phys. Chem. C 2013, 117, 16438–16447.
Tao, F.; Crozier, P. A. Atomic-scale observations of catalyst structures under reaction conditions and during catalysis. Chem. Rev. 2016, 116, 3487–3539.
Dou, J.; Sun, Z. C.; Opalade, A. A.; Wang, N.; Fu, W. S.; Tao, F. Operando chemistry of catalyst surfaces during catalysis. Chem. Soc. Rev. 2017, 46, 2001–2027.
Montano, M.; Tang, D. C.; Somorjai, G. A. Scanning tunneling microscopy (STM) at high pressures. Adsorption and catalytic reaction studies on platinum and rhodium single crystal surfaces. Catal. Lett. 2006, 107, 131–141.
Kim, J.; Noh, M. C.; Doh, W. H.; Park, J. Y. In situ observation of competitive Co and O2 adsorption on the Pt(111) surface using near-ambient pressure scanning tunneling microscopy. J. Phys. Chem. C 2018, 122, 6246–6254.
Vang, R. T.; Laegsgaard, E.; Besenbacher, F. Bridging the pressure gap in model systems for heterogeneous catalysis with high-pressure scanning tunneling microscopy. Phys. Chem. Chem. Phys. 2007, 9, 3460–3469.
Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio totalenergy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186.
Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50.
Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561.
Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799.
Zhao, P.; He, Y. R.; Cao, D. B.; Wen, X. D.; Xiang, H. W.; Li, Y. W.; Wang, J. G.; Jiao, H. J. High coverage adsorption and co-adsorption of CO and H2 on Ru(0001) from DFT and thermodynamics. Phys. Chem. Chem. Phys. 2015, 17, 19446–19456.
Brugger, T.; Ma, H. F.; Iannuzzi, M.; Berner, S.; Winkler, A.; Hutter, J.; Osterwalder, J.; Greber, T. Nanotexture switching of single-layer hexagonal boron nitride on rhodium by intercalation of hydrogen atoms. Angew. Chem., Int. Ed. 2010, 49, 6120–6124.
Sutter, P.; Albrecht, P.; Tong, X.; Sutter, E. Mechanical decoupling of graphene from Ru(0001) by interfacial reaction with oxygen. J. Phys. Chem. C 2013, 117, 6320–6324.
Ng, M. L.; Shavorskiy, A.; Rameshan, C.; Mikkelsen, A.; Lundgren, E.; Preobrajenski, A.; Bluhm, H. Reversible modification of the structural and electronic properties of a boron nitride monolayer by CO intercalation. ChemPhysChem 2015, 16, 923–927.
Dong, A. Y.; Fu, Q.; Wu, H.; Wei, M. M.; Bao, X. H. Factors controlling the CO intercalation of h-BN overlayers on Ru(0001). Phys. Chem. Chem. Phys. 2016, 18, 24278–24284.
Kidambi, P. R.; Blume, R.; Kling, J.; Wagner, J. B.; Baehtz, C.; Weatherup, R. S.; Schloegl, R.; Bayer, B. C.; Hofmann, S. In situ observations during chemical vapor deposition of hexagonal boron nitride on polycrystalline copper. Chem. Mater. 2014, 26, 6380–6392.
González-Herrero, H.; Pou, P.; Lobo-Checa, J.; Fernández-Torre, D.; Craes, F.; Martínez-Galera, A. J.; Ugeda, M. M.; Corso, M.; Ortega, J. E.; Gómez-Rodríguez, J. M. et al. Graphene tunable transparency to tunneling electrons: A direct tool to measure the local coupling. ACS Nano 2016, 10, 5131–5144.
Rutter, G. M.; Guisinger, N. P.; Crain, J. N.; Jarvis, E. A. A.; Stiles, M. D.; Li, T.; First, P. N.; Stroscio, J. A. Imaging the interface of epitaxial graphene with silicon carbide via scanning tunneling microscopy. Phys. Rev. B 2007, 76, 235416.
Brar, V. W.; Zhang, Y. B.; Yayon, Y.; Ohta, T.; McChesney, J. L.; Bostwick, A.; Rotenberg, E.; Horn, K.; Crommie, M. F. Scanning tunneling spectroscopy of inhomogeneous electronic structure in monolayer and bilayer graphene on SiC. Appl. Phys. Lett. 2007, 91, 122102.
Altfeder, I. B.; Chen, D. M.; Matveev, K. A. Imaging buried interfacial lattices with quantized electrons. Phys. Rev. Lett. 1998, 80, 4895–4898.
Longwitz, S. R.; Schnadt, J.; Vestergaard, E. K.; Vang, R. T.; Stensgaard, I.; Brune, H.; Besenbacher, F. High-coverage structures of carbon monoxide adsorbed on Pt(111) studied by high-pressure scanning tunneling microscopy. J. Phys. Chem. B 2004, 108, 14497–14502.
Tao, F.; Dag, S.; Wang, L.-W.; Liu, Z.; Butcher, D. R.; Bluhm, H.; Salmeron, M.; Somorjai, G. A. Break-up of stepped platinum catalyst surfaces by high CO coverage. Science 2010, 327, 850–853.
Jensen, J. A.; Rider, K. B.; Salmeron, M.; Somorjai, G. A. High pressure adsorbate structures studied by scanning tunneling microscopy: CO on Pt(111) in equilibrium with the gas phase. Phys. Rev. Lett. 1998, 80, 1228–1231.
Wakisaka, M.; Yoneyama, T.; Ashizawa, S.; Hyuga, Y.; Ohkanda, T.; Uchida, H.; Watanabe, M. Structural variations of CO adlayers on a Pt(100) electrode in 0.1 M HClO4 solution: An in situ STM study. Phys. Chem. Chem. Phys. 2013, 15, 11038–11047.
Lucas, C. A.; Markovic, N. M.; Ross, P. N. The adsorption and oxidation of carbon monoxide at the Pt(111)/electrolyte interface: Atomic structure and surface relaxation. Surf. Sci. 1999, 425, L381–L386.
Yoshimi, K.; Song, M.-B.; Ito, M. Carbon monoxide oxidation on a Pt(111) electrode studied by in-situ IRAS and STM: Coadsorption of CO with water on Pt(111). Surf. Sci. 1996, 368, 389–395.
Turro, N. J.; Wan, P. Photolysis of dibenzyl ketones adsorbed on zeolite molecular sieves. correlation of observed cage effects with carbonyl carbon-13 enrichment efficiencies. J. Am. Chem. Soc. 1985, 107, 678–682.
Zhang, Y. H.; Wang, X. Y.; Shan, W.; Wu, B. Y.; Fan, H. Z.; Yu, X. J.; Tang, Y.; Yang, P. Y. Enrichment of low-abundance peptides and proteins on zeolite nanocrystals for direct MALDI-TOF MS analysis. Angew. Chem., Int. Ed. 2005, 44, 615–617.
Li, Y. Y.; Perera, S. P.; Crittenden, B. D. Zeolite monoliths for air separation: Part 2: Oxygen enrichment, pressure drop and pressurization. Chem. Eng. Res. Des. 1998, 76, 931–941.
Guan, J.; Pan, X. L.; Liu, X.; Bao, X. H. Syngas segregation induced by confinement in carbon nanotubes: A combined first-principles and monte carlo study. J. Phys. Chem. C 2009, 113, 21687–21692.
Sun, M. M.; Dong, J. C.; Lv, Y.; Zhao, S. Q.; Meng, C. X.; Song, Y. J.; Wang, G. X.; Li, J. F.; Fu, Q.; Tian, Z. Q. et al. Pt@h-BN core–shell fuel cell electrocatalysts with electrocatalysis confined under outer shells. Nano Res. 2018, 11, 3490–3498.
Sun, M. M.; Fu, Q.; Gao, L. J.; Zheng, Y. P.; Li, Y. Y.; Chen, M. S.; Bao, X. H. Catalysis under shell: Improved CO oxidation reaction confined in Pt@h-BN core–shell nanoreactors. Nano Res. 2017, 10, 1403–1412.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 21688102, 21621063, 91545204, and 21703274), the Ministry of Science and Technology of China (No. 2016YFA0200200), and the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB17020000). The authors are grateful for the support for Nano-X from Suzhou Institute of Nano-Tech and Nano-Bionics, Chinese Academy of Sciences (SINANO) and discussions with Dr. Yang Yang and Dr. Haobo Li.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
12274_2018_2184_MOESM1_ESM.pdf
Dynamic nanoscale imaging of enriched CO adlayer on Pt(111) confined under h-BN monolayer in ambient pressure atmospheres
Rights and permissions
About this article
Cite this article
Wu, H., Ren, P., Zhao, P. et al. Dynamic nanoscale imaging of enriched CO adlayer on Pt(111) confined under h-BN monolayer in ambient pressure atmospheres. Nano Res. 12, 85–90 (2019). https://doi.org/10.1007/s12274-018-2184-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-018-2184-8