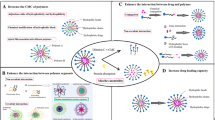
Abstract
Micelles have been studied as drug delivery carriers for decades. Their use can potentially result in high drug accumulation at the target site through the enhanced permeability and retention effect. Nevertheless, the lack of stability of micelles in the physiological environment limits their efficacy as a drug carrier. In particular, micelles tend to disassociate and prematurely release the encapsulated drugs, lowering delivery efficacy and creating toxicity concerns. Many efforts to enhance the stability of micelles have focused mainly on decreasing the critical micelle forming concentration and improving blood circulation. Herein, we review different strategies including crosslinking and non-crosslinking approaches designed to stabilize micelles and offer perspectives on future research directions.
Similar content being viewed by others
References
Verma, G.; Hassan, P. Self assembled materials: Design strategies and drug delivery perspectives. Phys. Chem. Chem. Phys. 2013, 15, 17016–17028.
Kamaly, N.; Xiao, Z. Y.; Valencia, P. M.; Radovic–Moreno, A. F.; Farokhzad, O. C. Targeted polymeric therapeutic nanoparticles: Design, development and clinical translation. Chem. Soc. Rev. 2012, 41, 2971–3010.
Service, R. F. Nanoparticle trojan horses gallop from the lab into the clinic. Science 2010, 330, 314–315.
Zhang, L.; Gu, F. X.; Chan, J. M.; Wang, A. Z.; Langer, R. S.; Farokhzad, O. C. Nanoparticles in medicine: Therapeutic applications and developments. Clin. Pharmacol. Therapeut. 2008, 83, 761–769.
Service, R. F. Nanotechnology takes aim at cancer. Science 2005, 310, 1132–1134.
Yoo, J. W.; Irvine, D. J.; Discher, D. E.; Mitragotri, S. Bio–inspired, bioengineered and biomimetic drug delivery carriers. Nat. Rev. Drug Discov. 2011, 10, 521–535.
Scheinberg, D. A.; Villa, C. H.; Escorcia, F. E.; McDevitt, M. R. Conscripts of the infinite armada: Systemic cancer therapy using nanomaterials. Nat. Rev. Clin. Oncol. 2010, 7, 266–276.
Petros, R. A.; De Simone, J. M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010, 9, 615–627.
Kim, S.; Kim, J. H.; Jeon, O.; Kwon, I. C.; Park, K. Engineered polymers for advanced drug delivery. Eur. J. Pharm. Biopharm. 2009, 71, 420–430.
Guo, S. T.; Huang, L. Nanoparticles containing insoluble drug for cancer therapy. Biotechnol. Adv. 2014, 32, 778–788.
Guo, S. T.; Miao, L.; Wang, Y. H.; Huang, L. Unmodified drug used as a material to construct nanoparticles: Delivery of cisplatin for enhanced anti–cancer therapy. J. Control. Release 2014, 174, 137–142.
Tong, R.; Tang, L.; Ma, L.; Tu, C. L.; Baumgartner, R.; Cheng, J. J. Smart chemistry in polymeric nanomedicine. Chem. Soc. Rev. 2014, 43, 6982–7012.
Hu, C. M. J.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R. H.; Zhang, L. F. Erythrocyte membrane–camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985.
Kulkarni, V. S.; Shaw, C. Chapter 2–Surfactants, lipids, and surface chemistry. In Essential Chemistry for Formulators of Semisolid and Liquid Dosages. V. S. Kulkarni; C. Shaw, Eds.; Academic Press: Boston, 2016; pp 5–19.
Vert, M.; Doi, Y.; Hellwich, K. H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schué, F. Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Pure Appl. Chem. 2012, 84, 377–410.
Cabral, H.; Matsumoto, Y.; Mizuno, K.; Chen, Q.; Murakami, M.; Kimura, M.; Terada, Y.; Kano, M. R.; Miyazono, K.; Uesaka, M. et al. Accumulation of sub–100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 2011, 6, 815–823.
Feng, X.; Wang, C. X.; Lin, B. R.; Xu, F. Methoxy poly(ethylene glycol)–conjugated linoleic acid polymeric micelles for paclitaxel delivery. Colloid J. 2006, 68, 779–783.
Kim, S.; Shi, Y. Z.; Kim, J. Y.; Park, K.; Cheng, J. X. Overcoming the barriers in micellar drug delivery: Loading efficiency, in vivo stability, and micelle–cell interaction. Expert Opin. Drug Deliv. 2010, 7, 49–62.
Rösler, A.; Vandermeulen, G. W. M.; Klok, H. A. Advanced drug delivery devices via self–assembly of amphiphilic block copolymers. Adv. Drug Deliv. Rev. 2001, 53, 95–108.
Alibolandi, M.; Ramezani, M.; Abnous, K.; Sadeghi, F.; Hadizadeh, F. Comparative evaluation of polymersome versus micelle structures as vehicles for the controlled release of drugs. J. Nanopart. Res. 2015, 17, 76.
Muthu, M. S.; Kulkarni, S. A.; Liu, Y. T.; Feng, S. S. Development of docetaxel–loaded vitamin E TPGS micelles: Formulation optimization, effects on brain cancer cells and biodistribution in rats. Nanomedicine 2012, 7, 353–364.
Fukushima, S.; Miyata, K.; Nishiyama, N.; Kanayama, N.; Yamasaki, Y.; Kataoka, K. PEGylated polyplex micelles from triblock catiomers with spatially ordered layering of condensed pDNA and buffering units for enhanced intracellular gene delivery. J. Am. Chem. Soc. 2005, 127, 2810–2811.
O’Reilly, R. K.; Hawker, C. J.; Wooley, K. L. Cross–linked block copolymer micelles: Functional nanostructures of great potential and versatility. Chem. Soc. Rev. 2006, 35, 1068–1083.
Fox, M. E.; Szoka, F. C.; Fréchet, J. M. J. Soluble polymer carriers for the treatment of cancer: The importance of molecular architecture. Acc. Chem. Res. 2009, 42, 1141–1151.
Venkatachalam, M. A.; Rennke, H. G. The structural and molecular basis of glomerular filtration. Circul. Res. 1978, 43, 337–347.
Jain, R. K. Transport of molecules across tumor vasculature. Cancer Metast. Rev. 1987, 6, 559–593.
Alexis, F.; Pridgen, E.; Molnar, L. K.; Farokhzad, O. C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharmaceutics 2008, 5, 505–515.
Owens, D. E.; Peppas, N. A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102.
Vonarbourg, A.; Passirani, C.; Saulnier, P.; Benoit, J. P. Parameters influencing the stealthiness of colloidal drug delivery systems. Biomaterials 2006, 27, 4356–4373.
Moghimi, S. M.; Hunter, A. C.; Murray, J. C. Long–circulating and target–specific nanoparticles: Theory to practice. Pharmacol. Rev. 2001, 53, 283–318.
Tang, L.; Yang, X. J.; Yin, Q.; Cai, K. M.; Wang, H.; Chaudhury, I.; Yao, C.; Zhou, Q.; Kwon, M.; Hartman, J. A. et al. Investigating the optimal size of anticancer nanomedicine. Proc. Natl. Acad. Sci. USA 2014, 111, 15344–15349.
Jiang, W.; Kim, B. Y. S.; Rutka, J. T.; Chan, W. C. W. Nanoparticle–mediated cellular response is size–dependent. Nat. Nanotechnol. 2008, 3, 145–150.
Duncan, R.; Sat, Y. N. Tumor targeting by enhanced permeability and retention (EPR) effect. Ann. Oncol. 1998, 9, 39.
Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284.
Stirland, D. L.; Nichols, J. W.; Miura, S.; Bae, Y. H. Mind the gap: A survey of how cancer drug carriers are susceptible to the gap between research and practice. J. Control. Release 2013, 172, 1045–1064.
http://www.doxil.com/doxil–supply–shortage.
Lamb, M.; Laugenour, K.; Liang, O. W.; Alexander, M.; Foster, C. E. I.; Lakey, J. R. T. In vitro maturation of viable islets from partially digested young pig pancreas. Cell Transplant. 2014, 23, 263–272.
Blanco, E.; Shen, H. F.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951.
Gao, Y.; Chen, L. L.; Gu, W. W.; Xi, Y.; Lin, L. P.; Li, Y. P. Targeted nanoassembly loaded with docetaxel improves intracellular drug delivery and efficacy in murine breast cancer model. Mol. Pharmaceutics 2008, 5, 1044–1054.
Feng, L.; Mumper, R. J. A critical review of lipid–based nanoparticles for taxane delivery. Cancer Lett. 2013, 334, 157–175.
Talelli, M.; Barz, M.; Rijcken, C. J. F.; Kiessling, F.; Hennink, W. E.; Lammers, T. Core–crosslinked polymeric micelles: Principles, preparation, biomedical applications and clinical translation. Nano Today 2015, 10, 93–117.
Dominguez, A.; Fernandez, A.; Gonzalez, N.; Iglesias, E.; Montenegro, L. Determination of critical micelle concentration of some surfactants by three techniques. J. Chem. Educ. 1997, 74, 1227.
Al–Soufi, W.; Piñeiro, L.; Novo, M. A model for monomer and micellar concentrations in surfactant solutions: Application to conductivity, NMR, diffusion, and surface tension data. J. Colloid Interface Sci. 2012, 370, 102–110.
Moroi, Y. Micelles: Theoretical and Applied Aspects; Springer Science & Business Media: New York, 1992.
Olesen, N. E.; Westh, P.; Holm, R. Determination of thermodynamic potentials and the aggregation number for micelles with the mass–action model by isothermal titration calorimetry: A case study on bile salts. J. Colloid Interface Sci. 2015, 453, 79–89.
Bouchemal, K.; Agnely, F.; Koffi, A.; Djabourov, M.; Ponchel, G. What can isothermal titration microcalorimetry experiments tell us about the self–organization of surfactants into micelles? J. Mol. Recogn. 2010, 23, 335–342.
Lu, J.; Owen, S. C.; Shoichet, M. S. Stability of self–assembled polymeric micelles in serum. Macromolecules 2011, 44, 6002–6008.
Chen, H. T.; Kim, S.; He, W.; Wang, H. F.; Low, P. S.; Park, K.; Cheng, J. X. Fast release of lipophilic agents from circulating PEG–PDLLA micelles revealed by in vivo Förster resonance energy transfer imaging. Langmuir 2008, 24, 5213–5217.
Chen, H. T.; Kim, S.; Li, L.; Wang, S. Y.; Park, K.; Cheng, J. X. Release of hydrophobic molecules from polymer micelles into cell membranes revealed by Forster resonance energy transfer imaging. Proc. Natl. Acad. Sci. USA 2008, 105, 6596–6601.
Xu, W.; Ling, P. X.; Zhang, T. M. Polymeric micelles, a promising drug delivery system to enhance bioavailability of poorly water–soluble drugs. J. Drug Deliv. 2013, 2013, 340315. DOI: 10.1155/2013/340315.
Topel, Ö.; Çakir, B. A.; Budama, L.; Hoda, N. Determination of critical micelle concentration of polybutadiene–blockpoly( ethyleneoxide) diblock copolymer by fluorescence spectroscopy and dynamic light scattering. J. Mol. Liq. 2013, 177, 40–43.
Adams, M. L.; Lavasanifar, A.; Kwon, G. S. Amphiphilic block copolymers for drug delivery. J. Pharm. Sci. 2003, 92, 1343–1355.
Avgoustakis, K. Pegylated poly(lactide) and poly(lactideco–glycolide) nanoparticles: Preparation, properties and possible applications in drug delivery. Curr. Drug Deliv. 2004, 1, 321–333.
Cheng, J. J.; Teply, B. A.; Sherifi, I.; Sung, J.; Luther, G.; Gu, F. X.; Levy–Nissenbaum, E.; Radovic–Moreno, A. F.; Langer, R.; Farokhzad, O. C. Formulation of functionalized PLGA–PEG nanoparticles for in vivo targeted drug delivery. Biomaterials 2007, 28, 869–876.
Astete, C. E.; Sabliov, C. M. Synthesis and characterization of PLGA nanoparticles. J. Biomater. Sci. Polym. Ed. 2006, 17, 247–289.
Önyüksel, H.; Jeon, E.; Rubinstein, I. Nanomicellar paclitaxel increases cytotoxicity of multidrug resistant breast cancer cells. Cancer Lett. 2009, 274, 327–330.
Thurmond, K. B.; Kowalewski, T.; Wooley, K. L. Watersoluble knedel–like structures: The preparation of shellcross–linked small particles. J. Am. Chem. Soc. 1996, 118, 7239–7240.
Bütün, V.; Billingham, N. C.; Armes, S. P. Synthesis of shell cross–linked micelles with tunable hydrophilic/hydrophobic cores. J. Am. Chem. Soc. 1998, 120, 12135–12136.
Bütün, V.; Wang, X. S.; de Paz Báñez, M. V.; Robinson, K. L.; Billingham, N. C.; Armes, S. P.; Tuzar, Z. Synthesis of shell cross–linked micelles at high solids in aqueous media. Macromolecules 2000, 33, 1–3.
Talelli, M.; Iman, M.; Varkouhi, A. K.; Rijcken, C. J. F.; Schiffelers, R. M.; Etrych, T.; Ulbrich, K.; van Nostrum, C. F.; Lammers, T.; Storm, G. et al. Core–crosslinked polymeric micelles with controlled release of covalently entrapped doxorubicin. Biomaterials 2010, 31, 7797–7804.
Ding, J. X.; Zhuang, X. L.; Xiao, C. S.; Cheng, Y. L.; Zhao, L.; He, C. L.; Tang, Z. H.; Chen, X. S. Preparation of photo–cross–linked pH–responsive polypeptide nanogels as potential carriers for controlled drug delivery. J. Mater. Chem. 2011, 21, 11383–11391.
Jin, Q.; Liu, X. S.; Liu, G. Y.; Ji, J. Fabrication of core or shell reversibly photo cross–linked micelles and nanogels from double responsive water–soluble block copolymers. Polymer 2010, 51, 1311–1319.
Ding, J. F.; Liu, G. J. Polystyrene–block–poly(2–cinnamoylethyl methacrylate) nanospheres with cross–linked shells. Macromolecules 1998, 31, 6554–6558.
Cohen, M. D.; Schmidt, G. M. J. Topochemistry. Part I. A survey. J. Chem. Soc. 1964, 1996–2000.
Schmidt, G. M. J. Topochemistry. Part III. The crystal chemistry of some trans–cinnamic acids. J. Chem. Soc. 1964, 2014–2021.
Yusa, S. I.; Sugahara, M.; Endo, T.; Morishima, Y. Preparation and characterization of a pH–responsive nanogel based on a photo–cross–linked micelle formed from block copolymers with controlled structure. Langmuir 2009, 25, 5258–5265.
Lendlein, A.; Jiang, H. Y.; Jünger, O.; Langer, R. Lightinduced shape–memory polymers. Nature 2005, 434, 879–882.
Xu, L.; Zhang, W. Y.; Cai, H. B.; Liu, F.; Wang, Y.; Gao, Y.; Zhang, W. A. Photocontrollable release and enhancement of photodynamic therapy based on host–guest supramolecular amphiphiles. J. Mater. Chem. B 2015, 3, 7417–7426.
Deepagan, V. G.; Kwon, S.; You, D. G.; Nguyen, V. Q.; Um, W.; Ko, H.; Lee, H.; Jo, D. G.; Kang, Y. M.; Park, J. H. In situ diselenide–crosslinked polymeric micelles for ROS–mediated anticancer drug delivery. Biomaterials 2016, 103, 56–66.
Zhai, S. D.; Hu, X. L.; Hu, Y. J.; Wu, B. Y.; Xing, D. Visible light–induced crosslinking and physiological stabilization of diselenide–rich nanoparticles for redox–responsive drug release and combination chemotherapy. Biomaterials 2017, 121, 41–54.
Zhang, Q.; Remsen, E. E.; Wooley, K. L. Shell cross–linked nanoparticles containing hydrolytically degradable, crystalline core domains. J. Am. Chem. Soc. 2000, 122, 3642–3651.
Huang, H. Y.; Kowalewski, T.; Remsen, E. E.; Gertzmann, R.; Wooley, K. L. Hydrogel–coated glassy nanospheres: A novel method for the synthesis of shell cross–linked knedels. J. Am. Chem. Soc. 1997, 119, 11653–11659.
Li, Y. T.; Lokitz, B. S.; McCormick, C. L. RAFT synthesis of a thermally responsive ABC triblock copolymer incorporating N–acryloxysuccinimide for facile in situ formation of shell cross–linked micelles in aqueous media. Macromolecules 2006, 39, 81–89.
Rodríguez–Hernández, J.; Babin, J.; Zappone, B.; Lecommandoux, S. Preparation of shell cross–linked nano–objects from hybrid–peptide block copolymers. Biomacromolecules 2005, 6, 2213–2220.
Pilon, L. N.; Armes, S. P.; Findlay, P.; Rannard, S. P. Synthesis and characterization of shell cross–linked micelles with hydroxy–functional coronas: A pragmatic alternative to dendrimers? Langmuir 2005, 21, 3808–3813.
Liu, S. Y.; Weaver, J. V. M.; Save, M.; Armes, S. P. Synthesis of pH–responsive shell cross–linked micelles and their use as nanoreactors for the preparation of gold nanoparticles. Langmuir 2002, 18, 8350–8357.
Zhang, J. Y.; Jiang, X. Z.; Zhang, Y. F.; Li, Y. T.; Liu, S. Y. Facile fabrication of reversible core cross–linked micelles possessing thermosensitive swellability. Macromolecules 2007, 40, 9125–9132.
Duong, H. T. T.; Nguyen, T. L. U.; Stenzel, M. H. Micelles with surface conjugated RGD peptide and crosslinked polyurea core via RAFT polymerization. Polym. Chem. 2010, 1, 171–182.
Huang, C. Q.; Hong, C. Y.; Pan, C. Y. Formation of flower–like aggregates from self–assembling of micelles with PEO shells and cross–linked polyacrylamide cores. Chin. J. Polym. Sci. 2008, 26, 341–352.
Shim, M. S.; Kwon, Y. J. Acid–transforming polypeptide micelles for targeted nonviral gene delivery. Biomaterials 2010, 31, 3404–3413.
Kim, J. S.; Youk, J. H. Preparation of core cross–linked micelles using a photo–cross–linking agent. Polymer 2009, 50, 2204–2208.
Joralemon, M. J.; O’Reilly, R. K.; Hawker, C. J.; Wooley, K. L. Shell click–crosslinked (SCC) nanoparticles: A new methodology for synthesis and orthogonal functionalization. J. Am. Chem. Soc. 2005, 127, 16892–16899.
Zhao, Y. Surface–cross–linked micelles as multifunctionalized organic nanoparticles for controlled release, light harvesting, and catalysis. Langmuir 2016, 32, 5703–5713.
Dai, Y.; Wang, H. Q.; Zhang, X. J. Reduction–responsive interlayer–crosslinked micelles prepared from star–shaped copolymer via click chemistry for drug controlled release. J. Nanopart. Res. 2017, 19, 383.
Withey, A. B.; Chen, G. J.; Nguyen, T. L. U.; Stenzel, M. H. Macromolecular cobalt carbonyl complexes encapsulated in a click–cross–linked micelle structure as a nanoparticle to deliver cobalt pharmaceuticals. Biomacromolecules 2009, 10, 3215–3226.
Jiang, X. Z.; Zhang, J. Y.; Zhou, Y. M.; Xu, J.; Liu, S. Y. Facile preparation of core–crosslinked micelles from azidecontaining thermoresponsive double hydrophilic diblock copolymer via click chemistry. J. Polym. Sci. Part A: Polym. Chem. 2008, 46, 860–871.
Du, J. Z.; Chen, Y. M.; Zhang, Y. H.; Han, C. C.; Fischer, K.; Schmidt, M. Organic/inorganic hybrid vesicles based on a reactive block copolymer. J. Am. Chem. Soc. 2003, 125, 14710–14711.
Du, J. Z.; Armes, S. P. pH–responsive vesicles based on a hydrolytically self–cross–linkable copolymer. J. Am. Chem. Soc. 2005, 127, 12800–12801.
Zhang, Y. F.; Gu, W. Y.; Xu, H. X.; Liu, S. Y. Facile fabrication of hybrid nanoparticles surface grafted with multi–responsive polymer brushes via block copolymer micellization and self–catalyzed core gelation. J. Polym. Sci. Part A: Polym. Chem. 2008, 46, 2379–2389.
Matsumoto, K.; Hasegawa, H.; Matsuoka, H. Synthesis of sodium–polystyrenesulfonate–grafted nanoparticles by corecross–linking of block copolymer micelles. Tetrahedron 2004, 60, 7197–7204.
Delgado, P. A.; Matloka, P.; Zuluaga, F.; Wagener, K. B. Synthesis and thermal crosslinking of carbosiloxane and oligo(oxyethylene) polymers. J. Polym. Sci. Part A: Polym. Chem. 2012, 50, 431–440.
Chen, W. X.; Cheng, Y. F.; Wang, B. H. Dual–responsive boronate crosslinked micelles for targeted drug delivery. Angew. Chem., Int. Ed. 2012, 51, 5293–5295.
Li, Y. P.; Xiao, W. W.; Xiao, K.; Berti, L.; Luo, J. T.; Tseng, H. P.; Fung, G.; Lam, K. S. Well–defined, reversible boronate crosslinked nanocarriers for targeted drug delivery in response to acidic pH values and cis–diols. Angew. Chem., Int. Ed. 2012, 51, 2864–2869.
Lin, V. S.; Dickinson, B. C.; Chang, C. J. Chapter two–Boronate–based fluorescent probes: Imaging hydrogen peroxide in living systems. Methods Enzymol. 2013, 526, 19–43.
Rhee, S. G. H2O2, a necessary evil for cell signaling. Science 2006, 312, 1882–1883.
Priftis, D.; Leon, L.; Song, Z. Y.; Perry, S. L.; Margossian, K. O.; Tropnikova, A.; Cheng, J. J.; Tirrell, M. Self–assembly of α–helical polypeptides driven by complex coacervation. Angew. Chem., Int. Ed. 2015, 54, 11128–11132.
Oberoi, H. S.; Laquer, F. C.; Marky, L. A.; Kabanov, A. V.; Bronich, T. K. Core cross–linked block ionomer micelles as pH–responsive carriers for cis–diamminedichloroplatinum(II). J. Control. Release 2011, 153, 64–72.
Harada, A.; Kataoka, K. Formation of polyion complex micelles in an aqueous milieu from a pair of oppositelycharged block copolymers with poly(ethylene glycol) segments. Macromolecules 1995, 28, 5294–5299.
Bütün, V.; Lowe, A. B.; Billingham, N. C.; Armes, S. P. Synthesis of zwitterionic shell cross–linked micelles. J. Am. Chem. Soc. 1999, 121, 4288–4289.
Zhang, X. H.; Ai, C. J.; Ma, J. H.; Xu, J.; Yang, S. G. Synthesis of zwitterionic shell cross–linked micelles with pH–dependent hydrophilicity. J. Colloid Interface Sci. 2011, 356, 24–30.
Dai, Y.; Wang, H. Q.; Zhang, X. J. Polyion complex micelles prepared by self–assembly of block–graft polycation and hyperbranched polyanion. J. Nanopart. Res. 2017, 19, 298.
Ueda, T.; Oshida, H.; Kurita, K.; Ishihara, K.; Nakabayashi, N. Preparation of 2–methacryloyloxyethyl phosphorylcholine copolymers with alkyl methacrylates and their blood compatibility. Polym. J. 1992, 24, 1259–1269.
Rungsardthong, U.; Deshpande, M.; Bailey, L.; Vamvakaki, M.; Armes, S. P.; Garnett, M. C.; Stolnik, S. Copolymers of amine methacrylate with poly(ethylene glycol) as vectors for gene therapy. J. Control. Release 2001, 73, 359–380.
Bronich, T. K.; Keifer, P. A.; Shlyakhtenko, L. S.; Kabanov, A. V. Polymer micelle with cross–linked ionic core. J. Am. Chem. Soc. 2005, 127, 8236–8237.
Shi, Y.; van Steenbergen, M. J.; Teunissen, E. A.; Novo, L.; Gradmann, S.; Baldus, M.; van Nostrum, C. F.; Hennink, W. E. Π–Π stacking increases the stability and loading capacity of thermosensitive polymeric micelles for chemotherapeutic drugs. Biomacromolecules 2013, 14, 1826–1837.
Shi, Y.; van der Meel, R.; Theek, B.; Oude Blenke, E.; Pieters, E. H. E.; Fens, M. H. A. M.; Ehling, J.; Schiffelers, R. M.; Storm, G.; van Nostrum, C. F. et al. Complete regression of xenograft tumors upon targeted delivery of paclitaxel via Π–Π stacking stabilized polymeric micelles. ACS Nano 2015, 9, 3740–3752.
Kim, S. H.; Tan, J. P. K.; Nederberg, F.; Fukushima, K.; Colson, J.; Yang, C.; Nelson, A.; Yang, Y. Y.; Hedrick, J. L. Hydrogen bonding–enhanced micelle assemblies for drug delivery. Biomaterials 2010, 31, 8063–8071.
Yang, C.; Ebrahim Attia, A. B.; Tan, J. P. K.; Ke, X. Y.; Gao, S. J.; Hedrick, J. L.; Yang, Y. Y. The role of non–covalent interactions in anticancer drug loading and kinetic stability of polymeric micelles. Biomaterials 2012, 33, 2971–2979.
Loh, X. J. Supramolecular host–guest polymeric materials for biomedical applications. Mater. Horiz. 2014, 1, 185–195.
Wang, J.; Jiang, M. Polymeric self–assembly into micelles and hollow spheres with multiscale cavities driven by inclusion complexation. J. Am. Chem. Soc. 2006, 128, 3703–3708.
Dong, X. P.; Guo, X. L.; Liu, G. Q.; Fan, A. P.; Wang, Z.; Zhao, Y. J. When self–assembly meets topology: An enhanced micelle stability. Chem. Commun. 2017, 53, 3822–3825.
Attwood, D.; Elworthy, P. H.; Kayne, S. B. Membrane osmometry of aqueous micellar solutions of pure nonionic and ionic surfactants. J. Phys. Chem. 1970, 74, 3529–3534.
Glavas, L.; Olsén, P.; Odelius, K.; Albertsson, A. C. Achieving micelle control through core crystallinity. Biomacromolecules 2013, 14, 4150–4156.
Li, F.; Danquah, M.; Mahato, R. I. Synthesis and characterization of amphiphilic lipopolymers for micellar drug delivery. Biomacromolecules 2010, 11, 2610–2620.
Ahmad, Z.; Shah, A.; Siddiq, M.; Kraatz, H. B. Polymeric micelles as drug delivery vehicles. RSC Adv. 2014, 4, 17028–17038.
Lavasanifar, A.; Samuel, J.; Kwon, G. S. The effect of alkyl core structure on micellar properties of poly(ethylene oxide)–block–poly(L–aspartamide) derivatives. Colloids Surf. B: Biointerfaces 2001, 22, 115–126.
Falamarzian, A.; Lavasanifar, A. Chemical modification of hydrophobic block in poly(ethylene oxide) poly (caprolactone) based nanocarriers: Effect on the solubilization and hemolytic activity of amphotericin B. Macromol. Biosci. 2010, 10, 648–656.
Choi, J.; Moquin, A.; Bomal, E.; Na, L.; Maysinger, D.; Kakkar, A. Telodendrimers for physical encapsulation and covalent linking of individual or combined therapeutics. Mol. Pharmaceutics 2017, 14, 2607–2615.
Brinkman, A. M.; Chen, G. J.; Wang, Y. D.; Hedman, C. J.; Sherer, N. M.; Havighurst, T. C.; Gong, S. Q.; Xu, W. Aminoflavone–loaded EGFR–targeted unimolecular micelle nanoparticles exhibit anti–cancer effects in triple negative breast cancer. Biomaterials 2016, 101, 20–31.
Chen, G. J.; Wang, L. W.; Cordie, T.; Vokoun, C.; Eliceiri, K. W.; Gong, S. Q. Multi–functional self–fluorescent unimolecular micelles for tumor–targeted drug delivery and bioimaging. Biomaterials 2015, 47, 41–50.
Lu, Y.; Yue, Z. G.; Xie, J. B.; Wang, W.; Zhu, H.; Zhang, E. S.; Cao, Z. Q. Micelles with ultralow critical micelle concentration as carriers for drug delivery. Nat. Biomed. Eng. 2018, 1, 318–325.
Cao, Z. Q.; Zhang, L.; Jiang, S. Y. Superhydrophilic zwitterionic polymers stabilize liposomes. Langmuir 2012, 28, 11625–11632.
Cao, Z. Q.; Jiang, S. Y. Super–hydrophilic zwitterionic poly(carboxybetaine) and amphiphilic non–ionic poly(ethylene glycol) for stealth nanoparticles. Nano Today 2012, 7, 404–413.
Yusa, S. I.; Fukuda, K.; Yamamoto, T.; Ishihara, K.; Morishima, Y. Synthesis of well–defined amphiphilic block copolymers having phospholipid polymer sequences as a novel biocompatible polymer micelle reagent. Biomacromolecules 2005, 6, 663–670.
Acknowledgements
This work was supported by the National Science Foundation (DMR-1410853) and National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (DP2DK111910).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, Y., Zhang, E., Yang, J. et al. Strategies to improve micelle stability for drug delivery. Nano Res. 11, 4985–4998 (2018). https://doi.org/10.1007/s12274-018-2152-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-018-2152-3