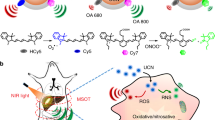
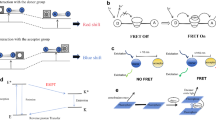
Abstract
Reactive oxygen and nitrogen species (RONS) play important roles in cell signal transduction. However, overproduction of RONS is associated with a series of pathological processes and may disrupt cellular homeostasis, causing oxidative and nitrosative stress. Accurate methods to selectively and specifically monitor RONS in living systems are required to further elucidate the biological functions of these species. Optical imaging possesses high sensitivity, high spatiotemporal resolution, and real-time imaging capability. These qualities are advantageous for the detection of RONS in living systems. This review summarizes the development of optical nanoprobes with near-infrared (NIR) fluorescent, upconversion luminescent, chemiluminescent, or photoacoustic signals for molecular imaging of RONS in living systems. In this review, we discuss the design principles and advantages of RONS-responsive activatable nanoprobes, as well as applications of these optical imaging modalities in different disease models.
Similar content being viewed by others
References
Dickinson, B. C.; Chang, C. J. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat. Chem. Biol. 2011, 7, 504–511.
D’Autréaux, B.; Toledano, M. B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824.
Nathan, C.; Cunningham–Bussel, A. Beyond oxidative stress: An immunologist’s guide to reactive oxygen species. Nat. Rev. Immunol. 2013, 13, 349–361.
Weseler, A. R.; Bast, A. Oxidative stress and vascular function: Implications for pharmacologic treatments. Curr. Hypertens. Rep. 2010, 12, 154–161.
Newsholme, P.; Cruzat, V. F.; Keane, K. N.; Carlessi, R.; de Bittencourt, P. I. H., Jr. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem. J. 2016, 473, 4527–4550.
Thornton, C.; Baburamani, A. A.; Kichev, A.; Hagberg, H. Oxidative stress and endoplasmic reticulum (ER) stress in the development of neonatal hypoxic–ischaemic brain injury. Biochem. Soc. Trans. 2017, 45, 1067–1076.
Wen, T.; Zhang, H.; Chong, Y.; Wamer, W. G.; Yin, J.–J.; Wu, X. C. Probing hydroxyl radical generation from H2O2 upon plasmon excitation of gold nanorods using electron spin resonance: Molecular oxygen–mediated activation. Nano Res. 2016, 9, 1663–1673.
Pravalika, K.; Sarmah, D.; Kaur, H.; Wanve, M.; Saraf, J.; Kalia, K.; Borah, A.; Yavagal, D. R.; Dave, K. R.; Bhattacharya, P. Myeloperoxidase and Neurological disorder: A crosstalk. ACS Chem. Neurosci. 2018, 9, 421–430.
Lundberg, J. O.; Gladwin, M. T.; Weitzberg, E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat. Rev. Drug Discov. 2015, 14, 623–641.
Schieber, M.; Chandel, N. S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462.
Weidinger, A.; Kozlov, A. V. Biological activities of reactive oxygen and nitrogen species: Oxidative stress versus signal transduction. Biomolecules 2015, 5, 472–484.
Gonçalves, N. P.; Vægter, C. B.; Andersen, H.; Østergaard, L.; Calcutt, N. A.; Jensen, T. S. Schwann cell interactions with axons and microvessels in diabetic neuropathy. Nat. Rev. Neurol. 2017, 13, 135–147.
Rani, V.; Deep, G.; Singh, R. K.; Palle, K.; Yadav, U. C. S. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193.
Gaki, G. S.; Papavassiliou, A. G. Oxidative stress–induced signaling pathways implicated in the pathogenesis of Parkinson’s disease. Neuromol. Med. 2014, 16, 217–230.
Sabharwal, S. S.; Schumacker, P. T. Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles' heel? Nat. Rev. Cancer 2014, 14, 709–721.
Van Gaal, L. F.; Mertens, I. L.; De Block, C. E. Mechanisms linking obesity with cardiovascular disease. Nature 2006, 444, 875–880.
Wojtala, A.; Bonora, M.; Malinska, D.; Pinton, P.; Duszynski, J.; Wieckowski, M. R. Methods to monitor ROS production by fluorescence microscopy and fluorometry. Methods Enzymol. 2014, 542, 243–262.
Giraldo, J. P.; Landry, M. P.; Faltermeier, S. M.; McNicholas, T. P.; Iverson, N. M.; Boghossian, A. A.; Reuel, N. F.; Hilmer, A. J.; Sen, F.; Brew, J. A. et al. Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nat. Mater. 2014, 13, 400–408.
Chen, G. Y.; Nuñez, G. Sterile inflammation: Sensing and reacting to damage. Nat. Rev. Immunol. 2010, 10, 826–837.
Heller, D. A.; Jin, H.; Martinez, B. M.; Patel, D.; Miller, B. M.; Yeung, T.–K.; Jena, P. V.; Höbartner, C.; Ha, T.; Silverman, S. K. et al. Multimodal optical sensing and analyte specificity using single–walled carbon nanotubes. Nat. Nanotechnol. 2009, 4, 114–120.
Miller, E. W.; Tulyathan, O.; Isacoff, E. Y.; Chang, C. J. Molecular imaging of hydrogen peroxide produced for cell signaling. Nat. Chem. Biol. 2007, 3, 263–267.
Chan, J.; Dodani, S. C.; Chang, C. J. Reaction–based smallmolecule fluorescent probes for chemoselective bioimaging. Nat. Chem. 2012, 4, 973–984.
Dickinson, B. C.; Srikun, D.; Chang, C. J. Mitochondrialtargeted fluorescent probes for reactive oxygen species. Curr. Opin. Chem. Biol. 2010, 14, 50–56.
Hyman, L. M.; Franz, K. J. Probing oxidative stress: Small molecule fluorescent sensors of metal ions, reactive oxygen species, and thiols. Coordin. Chem. Rev. 2012, 256, 2333–2356.
Yuan, L.; Lin, W. Y.; Zheng, K. B.; Zhu, S. S. FRET–based small–molecule fluorescent probes: Rational design and bioimaging applications. Acc. Chem. Res. 2013, 46, 1462–1473.
Urano, Y. Novel live imaging techniques of cellular functions and in vivo tumors based on precise design of small molecule–based “activatable” fluorescence probes. Curr. Opin. Chem. Biol. 2012, 16, 602–608.
Chen, X. Q.; Wang, F.; Hyun, J. Y.; Wei, T. W.; Qiang, J.; Ren, X. T.; Shin, I.; Yoon, J. Recent progress in the development of fluorescent, luminescent and colorimetric probes for detection of reactive oxygen and nitrogen species. Chem. Soc. Rev. 2016, 45, 2976–3016.
Kowada, T.; Maeda, H.; Kikuchi, K. BODIPY–based probes for the fluorescence imaging of biomolecules in living cells. Chem. Soc. Rev. 2015, 44, 4953–4972.
Kim, H. M.; Cho, B. R. Small–molecule two–photon probes for bioimaging applications. Chem. Rev. 2015, 115, 5014–5055.
Smith, A. M.; Duan, H. W.; Mohs, A. M.; Nie, S. M. Bioconjugated quantum dots for in vivo molecular and cellular imaging. Adv. Drug Deliv. Rev. 2008, 60, 1226–1240.
Zhou, J.; Liu, Z.; Li, F. Y. Upconversion nanophosphors for small–animal imaging. Chem. Soc. Rev. 2012, 41, 1323–1349.
Koo, H.; Huh, M. S.; Ryu, J. H.; Lee, D.–E.; Sun, I.–C.; Choi, K.; Kim, K.; Kwon, I. C. Nanoprobes for biomedical imaging in living systems. Nano Today 2011, 6, 204–220.
Sun, T. M.; Zhang, Y. S.; Pang, B.; Hyun, D. C.; Yang, M. X.; Xia, Y. N. Engineered nanoparticles for drug delivery in cancer therapy. Angew. Chem., Int. Ed. 2014, 53, 12320–12364.
Blanco, E.; Shen, H. F.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951.
Zhen, X.; Tao, Y.; An, Z. F.; Chen, P.; Xu, C. J.; Chen, R. F.; Huang, W.; Pu, K. Y. Ultralong phosphorescence of watersoluble organic nanoparticles for in vivo afterglow imaging. Adv. Mater. 2017, 29, 1606665.
Zhen, X.; Xie, C.; Pu, K. Y. Temperature–correlated afterglow of a semiconducting polymer nanococktail for imaging–guided photothermal therapy. Angew. Chem., Int. Ed. 2018, 57, 3938–3942.
Li, J. C.; Rao, J. H.; Pu, K. Y. Recent progress on semiconducting polymer nanoparticles for molecular imaging and cancer phototherapy. Biomaterials 2018, 155, 217–235.
Michalet, X.; Pinaud, F. F.; Bentolila, L. A.; Tsay, J. M.; Doose, S.; Li, J. J.; Sundaresan, G.; Wu, A. M.; Gambhir, S. S.; Weiss, S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 2005, 307, 538–544.
Chen, M. J.; Yin, M. Z. Design and development of fluorescent nanostructures for bioimaging. Prog. Polym. Sci. 2014, 39, 365–395.
Wu, C. F.; Hansen, S. J.; Hou, Q.; Yu, J. B.; Zeigler, M.; Jin, Y. H.; Burnham, D. R.; McNeill, J. D.; Olson, J. M.; Chiu, D. T. Design of highly emissive polymer dot bioconjugates for in vivo tumor targeting. Angew. Chem., Int. Ed. 2011, 50, 3430–3434.
Hu, S.–H.; Gao, X. H. Nanocomposites with spatially separated functionalities for combined imaging and magnetolytic therapy. J. Am. Chem. Soc. 2010, 132, 7234–7237.
Zhou, W.; Gao, X.; Liu, D. B.; Chen, X. Y. Gold nanoparticles for in vitro diagnostics. Chem. Rev. 2015, 115, 10575–10636.
Chan, M.–H.; Lin, H.–M. Preparation and identification of multifunctional mesoporous silica nanoparticles for in vitro and in vivo dual–mode imaging, theranostics, and targeted tracking. Biomaterials 2015, 46, 149–158.
Feng, T.; Ai, X. Z.; An, G. H.; Yang, P. P.; Zhao, Y. L. Charge–convertible carbon dots for imaging–guided drug delivery with enhanced in vivo cancer therapeutic efficiency. ACS Nano 2016, 10, 4410–4420.
Fan, Z.; Sun, L. M.; Huang, Y. J.; Wang, Y. Z.; Zhang, M. J. Bioinspired fluorescent dipeptide nanoparticles for targeted cancer cell imaging and real–time monitoring of drug release. Nat. Nanotechnol. 2016, 11, 388–394.
Chinen, A. B.; Guan, C. M.; Ferrer, J. R.; Barnaby, S. N.; Merkel, T. J.; Mirkin, C. A. Nanoparticle probes for the detection of cancer biomarkers, cells, and tissues by fluorescence. Chem. Rev. 2015, 115, 10530–10574.
Wolfbeis, O. S. An overview of nanoparticles commonly used in fluorescent bioimaging. Chem. Soc. Rev. 2015, 44, 4743–4768.
Lyu, Y.; Pu, K. Y. Recent advances of activatable molecular probes based on semiconducting polymer nanoparticles in sensing and imaging. Adv. Sci. 2017, 4, 1600481.
Pu, K. Y.; Shuhendler, A. J.; Rao, J. H. Semiconducting polymer nanoprobe for in vivo imaging of reactive oxygen and nitrogen species. Angew. Chem., Int. Ed. 2013, 52, 10325–10329.
Wu, L.; Wu, I.–C.; DuFort, C. C.; Carlson, M. A.; Wu, X.; Chen, L.; Kuo, C.–T.; Qin, Y. L.; Yu, J. B.; Hingorani, S. R. et al. Photostable ratiometric pdot probe for in vitro and in vivo imaging of hypochlorous acid. J. Am. Chem. Soc. 2017, 139, 6911–6918.
Yin, C.; Zhu, H. J.; Xie, C.; Zhang, L.; Chen, P.; Fan, Q. L.; Huang, W.; Pu, K. Y. Organic nanoprobe cocktails for multilocal and multicolor fluorescence imaging of reactive oxygen species. Adv. Funct. Mater. 2017, 27, 1700493.
Ju, J.; Chen, W. In situ growth of surfactant–free gold nanoparticles on nitrogen–doped graphene quantum dots for electrochemical detection of hydrogen peroxide in biological environments. Anal. Chem. 2015, 87, 1903–1910.
Gao, X.; Ding, C. Q.; Zhu, A. W.; Tian, Y. Carbondot–based ratiometric fluorescent probe for imaging and biosensing of superoxide anion in live cells. Anal. Chem. 2014, 86, 7071–7078.
Xu, H. X.; Suslick, K. S. Water–soluble fluorescent silver nanoclusters. Adv. Mater. 2010, 22, 1078–1082.
Chen, L.–Y.; Wang, C.–W.; Yuan, Z. Q.; Chang, H.–T. Fluorescent gold nanoclusters: Recent advances in sensing and imaging. Anal. Chem. 2015, 87, 216–229.
Chen, T. T.; Hu, Y. H.; Cen, Y.; Chu, X.; Lu, Y. A dualemission fluorescent nanocomplex of gold–cluster–decorated silica particles for live cell imaging of highly reactive oxygen species. J. Am. Chem. Soc. 2013, 135, 11595–11602.
Liu, Q.; Feng, W.; Yang, T. S.; Yi, T.; Li, F. Y. Upconversion luminescence imaging of cells and small animals. Nat. Protoc. 2013, 8, 2033–2044.
Joubert, M.–F. Photon avalanche upconversion in rare earth laser materials. Opt. Mater. 1999, 11, 181–203.
Wang, F.; Banerjee, D.; Liu, Y. S.; Chen, X. Y.; Liu, X. G. Upconversion nanoparticles in biological labeling, imaging, and therapy. Analyst 2010, 135, 1839–1854.
Cheng, L.; Wang, C.; Liu, Z. Upconversion nanoparticles and their composite nanostructures for biomedical imaging and cancer therapy. Nanoscale 2013, 5, 23–37.
Park, Y. I.; Lee, K. T.; Suh, Y. D.; Hyeon, T. Upconverting nanoparticles: A versatile platform for wide–field two–photon microscopy and multi–modal in vivo imaging. Chem. Soc. Rev. 2015, 44, 1302–1317.
Zhou, J.; Liu, Q.; Feng, W.; Sun, Y.; Li, F. Y. Upconversion luminescent materials: Advances and applications. Chem. Rev. 2015, 115, 395–465.
Chen, G. Y.; Ågren, H.; Ohulchanskyy, T. Y.; Prasad, P. N. Light upconverting core–shell nanostructures: Nanophotonic control for emerging applications. Chem. Soc. Rev. 2015, 44, 1680–1713.
Li, X. M.; Zhang, F.; Zhao, D. Y. Lab on upconversion nanoparticles: Optical properties and applications engineering via designed nanostructure. Chem. Soc. Rev. 2015, 44, 1346–1378.
Liu, X. W.; Deng, R. R.; Zhang, Y. H.; Wang, Y.; Chang, H. J.; Huang, L.; Liu, X. G. Probing the nature of upconversion nanocrystals: Instrumentation matters. Chem. Soc. Rev. 2015, 44, 1479–1508.
Dong, H.; Sun, L.–D.; Yan, C.–H. Energy transfer in lanthanide upconversion studies for extended optical applications. Chem. Soc. Rev. 2015, 44, 1608–1634.
Peng, J. J.; Xu, W.; Teoh, C. L.; Han, S. Y.; Kim, B.; Samanta, A.; Er, J. C.; Wang, L.; Yuan, L.; Liu, X. G. et al. High–efficiency in vitro and in vivo detection of Zn2+ by dye–assembled upconversion nanoparticles. J. Am. Chem. Soc. 2015, 137, 2336–2342.
Chen, Z. W.; Liu, Z.; Li, Z. H.; Ju, E. G.; Gao, N.; Zhou, L.; Ren, J. S.; Qu, X. G. Upconversion nanoprobes for efficiently in vitro imaging reactive oxygen species and in vivo diagnosing rheumatoid arthritis. Biomaterials 2015, 39, 15–22.
Fan, W. P.; Bu, W. B.; Shen, B.; He, Q. J.; Cui, Z. W.; Liu, Y. Y.; Zheng, X. P.; Zhao, K. L.; Shi, J. L. Intelligent MnO2 nanosheets anchored with upconversion nanoprobes for concurrent pH–/H2O2–responsive UCL imaging and oxygenelevated synergetic therapy. Adv. Mater. 2015, 27, 4155–4161.
Li, Z.; Liang, T.; Lv, S. W.; Zhuang, Q. G.; Liu, Z. H. A rationally designed upconversion nanoprobe for in vivo detection of hydroxyl radical. J. Am. Chem. Soc. 2015, 137, 11179–11185.
Peng, J. J.; Samanta, A.; Zeng, X.; Han, S. Y.; Wang, L.; Su, D. D.; Loong, D. T. B.; Kang, N. Y.; Park, S. J.; All, A. H. et al. Real–time in vivo hepatotoxicity monitoring through chromophore–conjugated photon–upconverting nanoprobes. Angew. Chem., Int. Ed. 2017, 56, 4165–4169.
Seo, Y. H.; Singh, A.; Cho, H.–J.; Kim, Y.; Heo, J.; Lim, C.–K.; Park, S. Y.; Jang, W.–D.; Kim, S. Rational design for enhancing inflammation–responsive in vivo chemiluminescence via nanophotonic energy relay to near–infrared AIE–active conjugated polymer. Biomaterials 2016, 84, 111–118.
Mao, D.; Wu, W. B.; Ji, S. L.; Chen, C.; Hu, F.; Kong, D. L.; Ding, D.; Liu, B. Chemiluminescence–guided cancer therapy using a chemiexcited photosensitizer. Chem 2017, 3, 991–1007.
Lim, C. K.; Lee, Y. D.; Na, J.; Oh, J. M.; Her, S.; Kim, K.; Choi, K.; Kim, S.; Kwon, I. C. Chemiluminescence–generating nanoreactor formulation for near–infrared imaging of hydrogen peroxide and glucose level in vivo. Adv. Funct. Mater. 2010, 20, 2644–2648.
Lee, D.; Khaja, S.; Velasquez–Castano, J. C.; Dasari, M.; Sun, C.; Petros, J.; Taylor, W. R.; Murthy, N. In vivo imaging of hydrogen peroxide with chemiluminescent nanoparticles. Nat. Mater. 2007, 6, 765–769.
Cho, S.; Hwang, O.; Lee, I.; Lee, G.; Yoo, D.; Khang, G.; Kang, P. M.; Lee, D. Chemiluminescent and antioxidant micelles as theranostic agents for hydrogen peroxide associated–inflammatory diseases. Adv. Funct. Mater. 2012, 22, 4038–4043.
Shuhendler, A. J.; Pu, K. Y.; Cui, L.; Uetrecht, J. P.; Rao, J. H. Real–time imaging of oxidative and nitrosative stress in the liver of live animals for drug–toxicity testing. Nat. Biotechnol. 2014, 32, 373–380.
Zhen, X.; Zhang, C. W.; Xie, C.; Miao, Q. Q.; Lim, K. L.; Pu, K. Y. Intraparticle energy level alignment of semiconducting polymer nanoparticles to amplify chemiluminescence for ultrasensitive in vivo imaging of reactive oxygen species. ACS Nano 2016, 10, 6400–6409.
Lee, Y.–D.; Lim, C.–K.; Singh, A.; Koh, J.; Kim, J.; Kwon, I. C.; Kim, S. Dye/peroxalate aggregated nanoparticles with enhanced and tunable chemiluminescence for biomedical imaging of hydrogen peroxide. ACS Nano 2012, 6, 6759–6766.
Yu, J. B.; Rong, Y.; Kuo, C.–T.; Zhou, X.–H.; Chiu, D. T. Recent advances in the development of highly luminescent semiconducting polymer dots and nanoparticles for biological imaging and medicine. Anal. Chem. 2017, 89, 42–56.
Wang, J. W.; Lv, F. T.; Liu, L. B.; Ma, Y. G.; Wang, S. Strategies to design conjugated polymer based materials for biological sensing and imaging. Coordin. Chem. Rev. 2018, 354, 135–154.
Nishihara, R.; Suzuki, H.; Hoshino, E.; Suganuma, S.; Sato, M.; Saitoh, T.; Nishiyama, S.; Iwasawa, N.; Citterio, D.; Suzuki, K. Bioluminescent coelenterazine derivatives with imidazopyrazinone C–6 extended substitution. Chem. Commun. 2015, 51, 391–394.
Li, P.; Liu, L.; Xiao, H. B.; Zhang, W.; Wang, L. L.; Tang, B. A new polymer nanoprobe based on chemiluminescence resonance energy transfer for ultrasensitive imaging of intrinsic superoxide anion in mice. J. Am. Chem. Soc. 2016, 138, 2893–2896.
Choi, H. S.; Gibbs, S. L.; Lee, J. H.; Kim, S. H.; Ashitate, Y.; Liu, F. B.; Hyun, H.; Park, G.; Xie, Y.; Bae, S. et al. Targeted zwitterionic near–infrared fluorophores for improved optical imaging. Nat. Biotechnol. 2013, 31, 148–153.
Hong, G. S.; Lee, J. C.; Robinson, J. T.; Raaz, U.; Xie, L. M.; Huang, N. F.; Cooke, J. P.; Dai, H. J. Multifunctional in vivo vascular imaging using near–infrared II fluorescence. Nat. Med. 2012, 18, 1841–1846.
Wang, L. V.; Hu, S. Photoacoustic tomography: In vivo imaging from organelles to organs. Science 2012, 335, 1458–1462.
Weber, J.; Beard, P. C.; Bohndiek, S. E. Contrast agents for molecular photoacoustic imaging. Nat. Methods 2016, 13, 639–650.
Kim, C.; Favazza, C.; Wang, L. V. In vivo photoacoustic tomography of chemicals: High–resolution functional and molecular optical imaging at new depths. Chem. Rev. 2010, 110, 2756–2782.
Xu, M. H.; Wang, L. V. Photoacoustic imaging in biomedicine. Rev. Sci. Instrum. 2006, 77, 041101.
De La Zerda, A.; Zavaleta, C.; Keren, S.; Vaithilingam, S.; Bodapati, S.; Liu, Z.; Levi, J.; Smith, B. R.; Ma, T.–J.; Oralkan, O. et al. Carbon nanotubes as photoacoustic molecular imaging agents in living mice. Nat. Nanotechnol. 2008, 3, 557–562.
De La Zerda, A.; Liu, Z.; Bodapati, S.; Teed, R.; Vaithilingam, S.; Khuri–Yakub, B. T.; Chen, X. Y.; Dai, H. J.; Gambhir, S. S. Ultrahigh sensitivity carbon nanotube agents for photoacoustic molecular imaging in living mice. Nano Lett. 2010, 10, 2168–2172.
Fan, Q. L.; Cheng, K.; Yang, Z.; Zhang, R. P.; Yang, M.; Hu, X.; Ma, X. W.; Bu, L. H.; Lu, X. M.; Xiong, X. X. et al. Perylene–diimide–based nanoparticles as highly efficient photoacoustic agents for deep brain tumor imaging in living mice. Adv. Mater. 2015, 27, 843–847.
Wang, J. X.; Chen, F.; Arconada–Alvarez, S. J.; Hartanto, J.; Yap, L.–P.; Park, R.; Wang, F.; Vorobyova, I.; Dagliyan, G.; Conti, P. S. et al. A nanoscale tool for photoacoustic–based measurements of clotting time and therapeutic drug monitoring of heparin. Nano Lett. 2016, 16, 6265–6271.
Zhen, X.; Zhang, J. J.; Huang, J. G.; Xie, C.; Miao, Q. Q.; Pu, K. Y. Macrotheranostic probe with disease–activated near–infrared fluorescence, photoacoustic, and photothermal signals for imaging–guided therapy. Angew. Chem., Int. Ed. 2018, 57, 7804–7808.
Chen, M.; Tang, S. H.; Guo, Z. D.; Wang, X. Y.; Mo, S. G.; Huang, X. Q.; Liu, G.; Zheng, N. F. Core–shell Pd@Au nanoplates as theranostic agents for in–vivo photoacoustic imaging, CT imaging, and photothermal therapy. Adv. Mater. 2014, 26, 8210–8216.
Yang, K.; Hu, L. L.; Ma, X. X.; Ye, S. Q.; Cheng, L.; Shi, X. Z.; Li, C. H.; Li, Y. G.; Liu, Z. Multimodal imaging guided photothermal therapy using functionalized graphene nanosheets anchored with magnetic nanoparticles. Adv. Mater. 2012, 24, 1868–1872.
Lovell, J. F.; Jin, C. S.; Huynh, E.; Jin, H. L.; Kim, C.; Rubinstein, J. L.; Chan, W. C. W.; Cao, W. G.; Wang, L. V.; Zheng, G. Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents. Nat. Mater. 2011, 10, 324–332.
Lyu, Y.; Fang, Y.; Miao, Q. Q.; Zhen, X.; Ding, D.; Pu, K. Y. Intraparticle molecular orbital engineering of semiconducting polymer nanoparticles as amplified theranostics for in vivo photoacoustic imaging and photothermal therapy. ACS Nano 2016, 10, 4472–4481.
Zhen, X.; Xie, C.; Jiang, Y. Y.; Ai, X. Z.; Xing, B. G.; Pu, K. Y. Semiconducting photothermal nanoagonist for remote–controlled specific cancer therapy. Nano Lett. 2018, 18, 1498–1505.
Xie, C.; Cheng, P. H.; Pu, K. Y. Synthesis of PEGylated semiconducting polymer amphiphiles for molecular photoacoustic imaging and guided therapy. Chem.—Eur. J., in press, DOI: 10.1002/chem.201705716.
Jiang, Y. Y.; Pu, K. Y. Advanced photoacoustic imaging applications of near–infrared absorbing organic nanoparticles. Small 2017, 13, 1700710.
Cui, D.; Xie, C.; Pu, K. Y. Development of semiconducting polymer nanoparticles for photoacoustic imaging. Macromol. Rapid Comm. 2017, 38, 1700125.
Lovell, J. F.; Liu, T. W. B.; Chen, J.; Zheng, G. Activatable photosensitizers for imaging and therapy. Chem. Rev. 2010, 110, 2839–2857.
Miao, Q. Q.; Pu, K. Y. Emerging designs of activatable photoacoustic probes for molecular imaging. Bioconjugate Chem. 2016, 27, 2808–2823.
Pu, K. Y.; Shuhendler, A. J.; Jokerst, J. V.; Mei, J. G.; Gambhir, S. S.; Bao, Z.; Rao, J. H. Semiconducting polymer nanoparticles as photoacoustic molecular imaging probes in living mice. Nat. Nanotechnol. 2014, 9, 233–239.
Yin, C.; Zhen, X.; Fan, Q. L.; Huang, W.; Pu, K. Y. Degradable semiconducting oligomer amphiphile for ratiometric photoacoustic imaging of hypochlorite. ACS Nano 2017, 11, 4174–4182.
Zhang, J. J.; Zhen, X.; Upputuri, P. K.; Pramanik, M.; Chen, P.; Pu, K. Y. Activatable photoacoustic nanoprobes for in vivo ratiometric imaging of peroxynitrite. Adv. Mater. 2017, 29, 1604764.
Chen, Q.; Liang, C.; Sun, X. Q.; Chen, J. W.; Yang, Z. J.; Zhao, H.; Feng, L. Z.; Liu, Z. H2O2–responsive liposomal nanoprobe for photoacoustic inflammation imaging and tumor theranostics via in vivo chromogenic assay. Proc. Natl. Acad. Sci. USA 2017, 114, 5343–5348.
Xie, C.; Zhen, X.; Lyu, Y.; Pu, K. Y. Nanoparticle Regrowth enhances photoacoustic signals of semiconducting macromolecular probe for in vivo imaging. Adv. Mater. 2017, 29, 1703693.
Huryn, D. M.; Resnick, L. O.; Wipf, P. Contributions of academic laboratories to the discovery and development of chemical biology tools. J. Med. Chem. 2013, 56, 7161–7176.
Li, L. L.; Ma, H. L.; Qi, G. B.; Zhang, D.; Yu, F. Q.; Hu, Z. Y.; Wang, H. Pathological–condition–driven construction of supramolecular nanoassemblies for bacterial infection detection. Adv. Mater. 2016, 28, 254–262.
Zhen, X.; Feng, X. H.; Xie, C.; Zheng, Y. J.; Pu, K. Y. Surface engineering of semiconducting polymer nanoparticles for amplified photoacoustic imaging. Biomaterials 2017, 127, 97–106.
Cremer, J. W.; Covert, P. A.; Parmentier, E. A.; Signorell, R. Direct measurement of photoacoustic signal sensitivity to aerosol particle size. J. Phys. Chem. Lett. 2017, 8, 3398–3403.
Jiang, Y. Y.; Pu, K. Y. Molecular fluorescence and photoacoustic imaging in the second near–infrared optical window using organic contrast agents. Adv. Biosyst. 2018, 2, 1700262.
Acknowledgements
K. P. thanks Nanyang Technological University (Start-Up grant: NTUSUG: M4081627.120) and Singapore Ministry of Education (Academic Research Fund Tier 1: RG133/15 M4011559 and 2015-T1-002-091; and Tier 2 MOE2016-T2-1-098) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhen, X., Pu, K. Development of optical nanoprobes for molecular imaging of reactive oxygen and nitrogen species. Nano Res. 11, 5258–5280 (2018). https://doi.org/10.1007/s12274-018-2135-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-018-2135-4