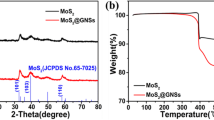
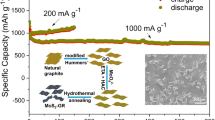
Abstract
Transition metal dichalcogenide nanodots (NDs) have received considerable interest. We report a facile bottom-up synthetic route for MoS2 NDs by using molybdenum pentachloride and L-cysteine as precursors in oleylamine. The synthesis of NDs with a narrow size distribution ranging from 2.2 to 5.3 nm, was tailored by controlling the reaction time. Because of its coating characteristics, oleyalmine leads to uniformity and monodispersity of the NDs. Moreover, the NDs synthesized have large specific surface areas providing active sites. Graphene possesses outstanding conductivity. Combining the advantages of the two materials, the 0D/2D material exhibits superior electrochemical performance because of the 2D permeable channels for ion adsorption, energy storage, and conversion. The as-prepared MoS2/rGO (~2.2 nm) showed a stable capacity of 220 mAh·g−1 after 10,000 cycles at the current density of 20 A·g−1. Furthermore, a reversible capacity ~140 mAh·g−1 was obtained at a much higher current density of 40 A·g−1. Additionally, this composite exhibited superior catalytic performance evidenced by a small overpotential (222 mV) to afford 10 mA·cm−2, and a small Tafel slope (59.8 mV·decade−1) with good acid-stability. The facile approach may pave the way for the preparation of NDs with these nanostructures for numerous applications.

Similar content being viewed by others
References
Li, X. L.; Li, Y. D. MoS2 nanostructures: Synthesis and electrochemical Mg2+ intercalation. J. Phys. Chem. B 2004, 108, 13893–13900.
Ayari, A.; Cobas, E.; Ogundadegbe, O.; Fuhrer, M. S. Realization and electrical characterization of ultrathin crystals of layered transition-metal dichalcogenides. J. Appl. Phys. 2007, 101, 014507.
Wang, H. T.; Tsai, C.; Kong D. S.; Chan, K. R.; Abild-Pedersen, F.; Nørskov, J. K.; Cui, Y. Transition-metal doped edge sites in vertically aligned MoS2 catalysts for enhanced hydrogen evolution. Nano Res. 2015, 8, 566–575.
Hu, Z.; Liu, Q. N.; Sun, W. Y.; Li, W. J.; Tao, Z. L.; Chou, S. L.; Chen, J.; Dou, S. X. MoS2 with an intercalation reaction as a long-life anode material for lithium ion batteries. Inorg. Chem. Front. 2016, 3, 532–535.
Guo, B. J.; Yu, K.; Li, H. L.; Song, H. L.; Zhang, Y. Y.; Lei, X.; Fu, H.; Tan, Y. H.; Zhu, Z. Q. Hollow structured micro/nano MoS2 spheres for high electrocatalytic activity hydrogen evolution reaction. ACS Appl. Mater. Interfaces 2016, 8, 5517–5525.
Ding, J. B.; Zhou, Y.; Li, Y. G.; Guo, S. J.; Huang, X. Q. MoS2 nanosheet assembling superstructure with a three-dimensional ion accessible site: A new class of bifunctional materials for batteries and electrocatalysis. Chem. Mater. 2016, 28, 2074–2080.
Gao, Q. S.; Giordano, C.; Antonietti, M. Biomimetic oxygen activation by MoS2/Ta3N5 nanocomposites for selective aerobic oxidation. Angew. Chem., Int. Ed. 2012, 51, 11740–11744.
Savjani, N.; Lewis, E. A.; Bissett, M. A.; Brent, J. R.; Dryfe, R. A. W.; Haigh, S. J.; O’Brien, P. Synthesis of lateral size-controlled monolayer 1H-MoS2@oleylamine as supercapacitor electrodes. Chem. Mater. 2016, 28, 657–664.
Xu, J. T.; Wang, M.; Wickramaratne, N. P.; Jaroniec, M.; Dou, S. X.; Dai, L. M. High-performance sodium ion batteries based on a 3D anode from nitrogen-doped graphene foams. Adv. Mater. 2015, 27, 2042–2048.
Xu, G. B.; Yang, L. W.; Wei, X. L.; Ding, J. W.; Zhong, J. X.; Chu, P. K. MoS2-quantum-dot-interspersed Li4Ti5O12 nanosheets with enhanced performance for Li-and Na-ion batteries. Adv. Funct. Mater. 2016, 26, 3349–3358.
Jin, H. L.; Huang, H. H.; He, Y. H.; Feng, X.; Wang, S.; Dai, L. M.; Wang, J. C. Graphene quantum dots supported by graphene nanoribbons with ultrahigh electrocatalytic performance for oxygen reduction. J. Am. Chem. Soc. 2015, 137, 7588–7591.
Bai, S.; Wang, L. M.; Chen, X. Y.; Du, J. T.; Xiong, Y. J. Chemically exfoliated metallic MoS2 nanosheets: A promising supporting co-catalyst for enhancing the photocatalytic performance of TiO2 nanocrystals. Nano Res. 2015, 8, 175–183.
Choi, M. S.; Qu, D. S.; Lee, D.; Liu, X. C.; Watanabe, K.; Taniguchi, T.; Yoo, W. J. Lateral MoS2 p-n junction formed by chemical doping for use in high-performance optoelectronics. ACS Nano 2014, 8, 9332–9340.
Ji, S. S.; Yang, Z.; Zhang, C.; Liu, Z. Y.; Tjiu, W. W.; Phang, I. Y.; Zhang, Z.; Pan, J. S.; Liu, T. X. Exfoliated MoS2 nanosheets as efficient catalysts for electrochemical hydrogen evolution. Electrochim. Acta 2013, 109, 269–275.
Gopalakrishnan, D.; Damien, D.; Shaijumon, M. M. MoS2 quantum dot-interspersed exfoliated MoS2 nanosheets. ACS Nano 2014, 8, 5297–5303.
Ren, X. P.; Pang, L. Q.; Zhang, Y. X.; Ren, X. D.; Fan, H. B.; Liu, S. Z. One-step hydrothermal synthesis of monolayer MoS2 quantum dots for highly efficient electrocatalytic hydrogen evolution. J. Mater. Chem. A 2015, 3, 10693–10697.
Zhu, C. B.; Mu, X. K.; van Aken, P. A.; Yu, Y.; Maier, J. Single-layered ultrasmallnanoplates of MoS2 embedded in carbon nanofibers with excellent electrochemical performance for lithium and sodium storage. Angew. Chem., Int. Ed. 2014, 53, 2152–2156.
Fan, X. L.; Chen, X. L.; Dai, L. M. 3D graphene based materials for energy storage. Curr. Opin. Colloid Interface Sci. 2015, 20, 429–438.
Kong, D. B.; He, H. Y.; Song, Q.; Wang, B.; Lv, W.; Yang, Q. H.; Zhi, L. J. Rational design of MoS2@graphene nanocables: Towards high performance electrode materials for lithium ion batteries. Energy Environ. Sci. 2014, 7, 3320–3325.
Choi, S. H.; Ko, Y. N.; Lee, J. K.; Kang, Y. C. 3D MoS2- graphene microspheres consisting of multiple nanospheres with superior sodium ion storage properties. Adv. Funct. Mater. 2015, 25, 1780–1788.
Li, Y. G.; Wang, H. L.; Xie, L. M.; Liang, Y. Y.; Hong, G. S.; Dai, H. J. MoS2 nanoparticles grown on graphene: An advanced catalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 2011, 133, 7296–7299.
Zhang, Z. Y.; Li, W. Y.; Yuen, M. F.; Ng, T.-W.; Tang, Y. B.; Lee, C.-S.; Chen, X. F.; Zhang, W. J. Hierarchical composite structure of few-layers MoS2 nanosheets supported by vertical graphene on carbon cloth for high-performance hydrogen evolution reaction. Nano Energy 2015, 18, 196–204.
Schniepp, H. C.; Li, J.-L.; McAllister, M. J.; Sai, H.; Herrera-Alonso, M.; Adamson, D. H.; Prud’homme, R. K.; Car, R.; Saville, D. A.; Aksay, I. A. Functionalized single graphene sheets derived from splitting graphite oxide. J. Phys. Chem. B 2006, 110, 8535–8539.
Hu, Z.; Wang, L. X.; Zhang, K.; Wang, J. B.; Cheng, F. Y.; Tao, Z. L.; Chen, J. MoS2 nanoflowers with expanded interlayers as high-performance anodes for sodium-ion batteries. Angew. Chem., Int. Ed. 2014, 53, 12794–12798.
Kwon, S. G.; Piao, Y. Z.; Park, J.; Angappane, S.; Jo, Y.; Hwang, N. M.; Park, J. G.; Hyeon, T. Kinetics of monodisperse iron oxide nanocrystal formation by “heating-up” process. J. Am. Chem. Soc. 2007, 129, 12571–12584.
Park, J.; An, K.; Hwang, Y.; Park, J. G.; Noh, H. J.; Kim, J. Y.; Park, J. H.; Hwang, N. M.; Hyeon, T. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat. Mater. 2004, 3, 891–895.
Xie, J. F.; Zhang, J. J.; Li, S.; Grote, F.; Zhang, X. D.; Zhang, H.; Wang, R. X.; Lei, Y.; Pan, B. C.; Xie, Y. Controllable disorder engineering in oxygen-incorporated MoS2 ultrathin nanosheets for efficient hydrogen evolution. J. Am. Chem. Soc. 2013, 135, 17881–17888.
Chang, K.; Chen, W. X. Single-layer MoS2/graphene dispersed in amorphous carbon: Towards high electrochemical performances in rechargeable lithium ion batteries. J. Mater. Chem. 2011, 21, 17175–17184.
Peng, S. J.; Liang, Y. L.; Cheng, F. Y.; Liang, J. Sizecontrolled chalcopyrite CuInS2 nanocrystals: One-pot synthesis and optical characterization. Sci. China Chem. 2012, 55, 1236–1241.
Xu, C.; Peng, S. J.; Tan, C. L.; Ang, H. X.; Tan, H. T.; Zhang, H.; Yan, Q. Y. Ultrathin S-doped MoSe2 nanosheets for efficient hydrogen evolution. J. Mater. Chem. A 2014, 2, 5597–5601.
Kwon, S. G.; Hyeon, T. Formation mechanisms of uniform nanocrystals via hot-injection and heat-up methods. Small 2011, 7, 2685–2702.
Zhao, F.; Sun, H. L.; Su, G.; Gao, S. Synthesis and size-dependent magnetic properties of monodisperse- EuSnanocrystals. Small 2006, 2, 244–248.
Williams, P. M.; Shakesheff, K. M.; Davies, M. C.; Jackson, D. E.; Roberts, C. J.; Tendler, S. J. B. Blind reconstruction of scanning probe image data. J. Vac. Sci. Technol. B 1996, 14, 1557–1562.
Zeng, Z. Y.; Yin, Z. Y.; Huang, X.; Li, H.; He, Q. Y.; Lu, G.; Boey, F.; Zhang, H. Single-layer semiconducting nanosheets: High-yield preparation and device fabrication. Angew. Chem., Int. Ed. 2011, 50, 11093–11097.
Du, J.; Chen, C. C.; Cheng, F. Y.; Chen, J. Rapid synthesis and efficient electrocatalytic oxygen reduction/evolution reaction of CoMn2O4nanodots supported on graphene. Inorg. Chem. 2015, 54, 5467–5474.
Zhang, K.; Kim, H. J.; Shi, X. J.; Lee, J. T.; Choi, J. M.; Song, M. S.; Park, J. H. Graphene/acid coassisted synthesis of ultrathin MoS2 nanosheets with outstanding rate capability for a lithium battery anode. Inorg. Chem. 2013, 52, 9807–9812.
Wang, Z.; Chen, T.; Chen, W. X.; Chang, K.; Ma, L.; Huang, G. C.; Chen, D. Y.; Lee, J. Y. CTAB-assisted synthesis of single-layer MoS2-graphene composites as anode materials of Li-ion batteries. J. Mater. Chem. A 2013, 1, 2202–2210.
Buscema, M.; Steele, G. A.; Van der Zant, H. S. J.; Castellanos-Gomez, A. The effect of the substrate on the Raman and photoluminescence emission of single-layer MoS2. Nano Res. 2014, 7, 561–571.
Splendiani, A.; Sun, L.; Zhang, Y. B.; Li, T. S.; Kim, J.; Chim, C. Y.; Galli, G.; Wang, F. Emerging photoluminescence in monolayer MoS2. Nano Lett. 2010, 10, 1271–1275.
Ma, C. B.; Qi, X. Y.; Chen, B.; Bao, S. Y.; Yin, Z. Y.; Wu, X. J.; Luo, Z. M.; Wei, J.; Zhang, H. L.; Zhang, H. MoS2 nanoflower-decorated reduced graphene oxide paper for high-performance hydrogen evolution reaction. Nanoscale 2014, 6, 5624–5629.
Liu, Y. T.; Zhu, X. D.; Duan, Z. Q.; Xie, X. M. Flexible and robust MoS2-graphene hybrid paper cross-linked by a polymer ligand: A high-performance anode material for thin film lithium-ion batteries. Chem. Commun. 2013, 49, 10305–10307.
Zhou, H. Q.; Wang, Y. M.; He, R.; Yu, F.; Sun, J. Y.; Wang, F.; Lan, Y. C.; Ren, Z. F.; Chen, S. One-step synthesis of self-supported porousNiSe2/Ni hybrid foam: An efficient 3D electrode for hydrogen evolution reaction. Nano Energy 2016, 20, 29–36.
Zhu, H.; Lyu, F.; Du, M. L.; Zhang, M.; Wang, Q. F.; Yao, J. M.; Guo, B. C. Design of two-dimensional, ultrathin MoS2 nanoplates fabricated within one-dimensional carbon nanofibers with thermosensitive morphology: High-performance electrocatalysts for the hydrogen evolution reaction. ACS Appl. Mater. Interfaces 2014, 6, 22126–22137.
Conway, B. E.; Tilak, B. V. Interfacial processes involving electrocatalytic evolution and oxidation of H2, and the role chemisorbed H. Electrochim. Acta 2002, 47, 3571–3594.
Zheng, X. L.; Xu, J. B.; Yan, K. Y.; Wang, H.; Wang, Z. L.; Yang, S. H. Space-confined growth of MoS2 nanosheets within graphite: The layered hybrid of MoS2 and graphene as an active catalyst for hydrogen evolution reaction. Chem. Mater. 2014, 26, 2344–2353.
Li, J. Y.; Hou, Y.; Gao, X. F.; Guan, D. S.; Xie, Y. Y.; Chen, J. H.; Yuan, C. A three-dimensionally interconnected carbon nanotube/layered MoS2 nanohybrid network for lithium ion battery anode with superior rate capacity and longcycle-life. Nano Energy 2015, 16, 10–18.
Lu, Y. Y.; Zhao, Q.; Zhang, N.; Lei, K. X.; Li, F. J.; Chen, J. Facile spraying synthesis and high-performance sodium storage of mesoporous MoS2/C microspheres. Adv. Funct. Mater. 2016, 26, 911–918.
Choi, S. H.; Kang, Y. C. Polystyrene-templated aerosol synthesis of MoS2-amorphous carbon composite with open macropores as battery electrode. ChemSusChem 2015, 8, 2260–2267.
Youn, D. H.; Jo, C.; Kim, J. Y.; Lee, J.; Lee, J. S. Ultrafast synthesis of MoS2 or WS2-reduced graphene oxide composites via hybrid microwave annealing for anode materials of lithium ion batteries. J. Power Sources 2015, 295, 228–234.
Liu, J.; Lu, P.-J.; Liang, S. Q.; Liu, J.; Wang, W. J.; Lei, M.; Tang, S. S.; Yang, Q. Ultrathin Li3VO4 nanoribbon/graphene sandwich-like nanostructures with ultrahigh lithium ion storage properties. Nano Energy 2015, 12, 709–724.
Wang, H.; Lan, X. Z.; Jiang, D. L.; Zhang, Y.; Zhong, H. H.; Zhang, Z. P.; Jiang, Y. Sodium storage and transport properties in pyrolysis synthesized MoSe2 nanoplates for high performance sodium-ion batteries. J. Power Sources 2015, 283, 187–194.
Douglas, A.; Carter, R.; Oakes, L.; Share, K.; Cohn, A. P.; Pint, C. L. Ultrafine iron pyrite (FeS2) nanocrystals improve sodium-sulfur and lithium-sulfur conversion reactions for efficient batteries. ACS Nano 2015, 9, 11156–11165.
Py, M. A.; Haering, R. R. Structural destabilization induced by lithium intercalation in MoS2 and related compounds. Can. J. Phys. 1983, 61, 76.
Liu, Y. C.; Zhang, N.; Jiao, L. F.; Tao, Z. L.; Chen, J. Ultrasmall Sn nanoparticles embedded in carbon as highperformance anode for sodium-ion batteries. Adv. Funct. Mater. 2015, 25, 214–220.
David, L.; Bhandavat, R.; Singh, G. MoS2/graphene composite paper for sodium-ion battery electrodes. ACS Nano 2014, 8, 1759–1770.
Acknowledgements
This work was supported by the National Key R&D Program (No. 2016YFB0901502), National Natural Science Foundation of China (Nos. 51231003, 51271094, and 21231005), Ministry of Education (Nos. B12015 and IRT13R30), and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
12274_2016_1410_MOESM1_ESM.pdf
Size-controlled MoS2 nanodots supported on reduced graphene oxide for hydrogen evolution reaction and sodium-ion batteries
Rights and permissions
About this article
Cite this article
Sun, W., Li, P., Liu, X. et al. Size-controlled MoS2 nanodots supported on reduced graphene oxide for hydrogen evolution reaction and sodium-ion batteries. Nano Res. 10, 2210–2222 (2017). https://doi.org/10.1007/s12274-016-1410-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-016-1410-5