Abstract
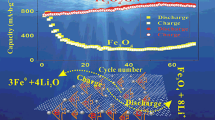
Sandwich structured graphene-wrapped FeS-graphene nanoribbons (G@FeS-GNRs) were developed. In this composite, FeS nanoparticles were sandwiched between graphene and graphene nanoribbons. When used as anodes in lithium ion batteries (LIBs), the G@FeS-GNR composite demonstrated an outstanding electrochemical performance. This composite showed high reversible capacity, good rate performance, and enhanced cycling stability owing to the synergy between the electrically conductive graphene, graphene nanoribbons, and FeS. The design concept developed here opens up a new avenue for constructing anodes with improved electrochemical stability for LIBs.

Similar content being viewed by others
References
Wei, W. F.; Cui, X. W.; Chen, W. X.; Ivey, D. G. Manganese oxide-based materials as electrochemical supercapacitor electrodes. Chem. Soc. Rev. 2011, 40, 1697–1721.
Zhu, J. X.; Yang, D.; Yin, Z. Y.; Yan, Q. Y.; Zhang, H. Graphene and graphene-based materials for energy storage applications. Small 2014, 10, 3480–3498.
Su, Y. Z.; Li, S.; Wu, D. Q.; Zhang, F.; Liang, H. W.; Gao, P. F.; Cheng, C.; Feng, X. L. Two-dimensional carbon-coated graphene/metal oxide hybrids for enhanced lithium storage. ACS Nano 2012, 6, 8349–8356.
Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262.
Tarascon, J. M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367.
Wang, B.; Li, X. L.; Zhang, X. F.; Luo, B.; Jin, M. H.; Liang, M. H.; Dayeh, S. A.; Picraux, S. T.; Zhi, L. J. Adaptable silicon-carbon nanocables sandwiched between reduced graphene oxide sheets as lithium ion battery anodes. ACS Nano 2013, 7, 1437–1445.
Whittingham, M. S. Lithium batteries and cathode materials. Chem. Rev. 2004, 104, 4271–4302.
Wang, H. L.; Dai, H. J. Strongly coupled inorganic-nanocarbon hybrid materials for energy storage. Chem. Soc. Rev. 2013, 42, 3088–3113.
Chang, K.; Chen, W. X. L-cysteine-assisted synthesis of layered MoS2/graphene composites with excellent electrochemical performances for lithium ion batteries. ACS Nano 2011, 5, 4720–4728.
Su, Q. M.; Xie, J.; Zhang, J.; Zhong, Y. J.; Du, G. H.; Xu, B. S. In situ transmission electron microscopy observation of electrochemical behavior of CoS2 in lithium-ion battery. ACS Appl. Mater. Interfaces 2014, 6, 3016–3022.
Su, C. W.; Li, J.-M.; Yang, W.; Guo, J. M. Electrodeposition of Nis3S2/Ni composites as high-performance cathodes for lithium batteries. J. Phys. Chem. C 2014, 118, 767–773.
Stephenson, T.; Li, Z.; Olsen, B.; Mitlin, D. Lithium ion battery applications of molybdenum disulfide (MoS2) nanocomposites. Energy Environ. Sci. 2014, 7, 209–231.
Zheng, S. Y.; Chen, Y.; Xu, Y. H.; Yi, F.; Zhu, Y. J.; Liu, Y. H.; Yang, J. H.; Wang, C. S. In situ formed lithium sulfide/microporous carbon cathodes for lithium-ion batteries. ACS Nano 2013, 7, 10995–11003.
Xu, C.; Zeng, Y.; Rui, X. H.; Xiao, N.; Zhu, J. X.; Zhang, W. Y.; Chen, J.; Liu, W. L.; Tan, H. T.; Hng, H. H. et al. Controlled soft-template synthesis of ultrathin C@FeS nanosheets with high-Li-storage performance. ACS Nano 2012, 6, 4713–4721.
Wu, B.; Song, H. H.; Zhou, J. S.; Chen, X. H. Iron sulfideembedded carbon microsphere anode material with high-rate performance for lithium-ion batteries. Chem. Commun. 2011, 47, 8653–8655.
Fei, L.; Lin, Q. L.; Yuan, B.; Chen, G.; Xie, P.; Li, Y. L.; Xu, Y.; Deng, S. G.; Smirnov, S.; Luo, H. M. Reduced graphene oxide wrapped FeS nanocomposite for lithium-ion battery anode with improved performance. ACS Appl. Mater. Interfaces 2013, 5, 5330–5335.
Wang, X. F.; Xiang, Q. Y.; Liu, B.; Wang, L. J.; Luo, T.; Chen, D.; Shen, G. Z. TiO2 modified FeS nanostructures with enhanced electrochemical performance for lithium-ion batteries. Sci. Rep. 2013, 3, 2007.
Li, L.; Raji, A. R. O.; Tour, J. M. Graphene-wrapped MnO2-graphene nanoribbons as anode materials for highperformance lithium ion batteries. Adv. Mater. 2013, 25, 6298–6302.
Genorio, B.; Lu, W.; Dimiev, A. M.; Zhu, Y.; Raji, A. R. O.; Novosel, B.; Alemany, L. B.; Tour, J. M. In situ intercalation replacement and selective functionalization of graphene nanoribbon stacks. ACS Nano 2012, 6, 4231–4240.
Campos-Delgado, J.; Romo-Herrera, J. M.; Jia, X. T.; Cullen, D. A.; Muramatsu, H.; Kim, Y. A.; Hayashi, T.; Ren, Z. F.; Smith, D. J.; Okuno, Y. et al. Bulk production of a new form of sp2 carbon: Crystalline graphene nanoribbons. Nano Lett. 2008, 8, 2773–2778.
Dong, C. C.; Zheng, X. D.; Huang, B.; Lu, M. Enhanced electrochemical performance of FeS coated by Ag as anode for lithium-ion batteries. Appl. Surf. Sci. 2013, 265, 114–119.
Saadat, S.; Tay, Y. Y.; Zhu, J. X.; Teh, P. F.; Maleksaeedi, S.; Shahjamali, M. M.; Shakerzadeh, M.; Srinivasan, M.; Tay, B. Y.; Hng, H. H. et al. Template-free electrochemical deposition of interconnected ZnSb nanoflakes for Li-ion battery anodes. Chem. Mater. 2011, 23, 1032–1038.
Kim, Y.; Goodenough, J. B. Lithium insertion into transitionmetal monosulfides: Tuning the position of the metal 4s band. J. Phys. Chem. C 2008, 112, 15060–15064.
Golodnitsky, D.; Peled, E. Pyrite as cathode insertion material in rechargeable lithium/composite polymer electrolyte batteries. Electrochim. Acta 1999, 45, 335–350.
Lai, H.; Li, J. X.; Chen, Z. G.; Huang, Z. G. Carbon nanohorns As a high-performance carrier for MnO2 anode in lithium-ion batteries. ACS Appl. Mater. Interfaces 2012, 4, 2325–2328.
Seo, J.-W.; Jun, Y.-W.; Park, S. W.; Nah, H.; Moon, T.; Park, B.; Kim, J.-G.; Kim, Y. J.; Cheon, J. Two-dimensional nanosheet crystals. Angew. Chem., Int. Ed. 2007, 46, 8828–8831.
Kim, B. C.; Takada, K.; Ohta, N.; Seino, Y.; Zhang, L. Q.; Wada, H.; Sasaki, T. All solid state Li-ion secondary battery with FeS anode. Solid State Ionics 2005, 176, 2383–2387.
Qian, D. N.; Xu, B.; Cho, H.-M.; Hatsukade, T.; Carroll, K. J.; Meng, Y. S. Lithium lanthanum titanium oxides: A fast ionic conductive coating for lithium-ion battery cathodes. Chem. Mater. 2012, 24, 2744–2751.
Yang, S. B.; Feng, X. L.; Zhi, L. J.; Cao, Q.; Maier, J.; Mü llen, K. Nanographene-constructed hollow carbon spheres and their favorable electroactivity with respect to lithium storage. Adv. Mater. 2010, 22, 838–842.
Han, H.; Song, T.; Lee, E. K.; Devadoss, A.; Jeon, Y.; Ha, J.; Chung, Y.-C.; Choi, Y.-M.; Jung, Y.-G.; Paik, U. Dominant factors governing the rate capability of a TiO2 nanotube anode for high power lithium ion batteries. ACS Nano 2012, 6, 8308–8315.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Li, L., Gao, C., Kovalchuk, A. et al. Sandwich structured graphene-wrapped FeS-graphene nanoribbons with improved cycling stability for lithium ion batteries. Nano Res. 9, 2904–2911 (2016). https://doi.org/10.1007/s12274-016-1175-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-016-1175-x