Abstract
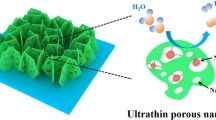
Exploring bifunctional catalysts for the hydrogen and oxygen evolution reactions (HER and OER) with high efficiency, low cost, and easy integration is extremely crucial for future renewable energy systems. Herein, ternary NiCoP nanosheet arrays (NSAs) were fabricated on 3D Ni foam by a facile hydrothermal method followed by phosphorization. These arrays serve as bifunctional alkaline catalysts, exhibiting excellent electrocatalytic performance and good working stability for both the HER and OER. The overpotentials of the NiCoP NSA electrode required to drive a current density of 50 mA/cm2 for the HER and OER are as low as 133 and 308 mV, respectively, which is ascribed to excellent intrinsic electrocatalytic activity, fast electron transport, and a unique superaerophobic structure. When NiCoP was integrated as both anodic and cathodic material, the electrolyzer required a potential as low as ~1.77 V to drive a current density of 50 mA/cm2 for overall water splitting, which is much smaller than a reported electrolyzer using the same kind of phosphide-based material and is even better than the combination of Pt/C and Ir/C, the best known noble metal-based electrodes. Combining satisfactory working stability and high activity, this NiCoP electrode paves the way for exploring overall water splitting catalysts.

Similar content being viewed by others
References
Luo, J.; Im, J. H.; Mayer, M. T.; Schreier, M.; Nazeeruddin, M. K.; Park, N. G.; Tilley, S. D.; Fan, H. J.; Gratzel, M. Water photolysis at 12.3% efficiency via perovskite photovoltaics and Earth-abundant catalysts. Science 2014, 345, 1593–1596.
Liu, J.; Liu, Y.; Liu, N. Y.; Han, Y. Z.; Zhang, X.; Huang, H.; Lifshitz, Y.; Lee, S. T.; Zhong, J.; Kang, Z. H. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 2015, 347, 970–974.
Tang, C.; Cheng, N. Y.; Pu, Z. H.; Xing, W.; Sun, X. P. NiSe nanowire film supported on nickel foam: An efficient and stable 3D bifunctional electrode for full water splitting. Angew. Chem., Int. Ed. 2015, 54, 9351–9355.
Wang, H. T.; Lee, H. W.; Deng, Y.; Lu, Z. Y.; Hsu, P. C.; Liu, Y. Y.; Lin, D. C.; Cui, Y. Bifunctional non-noble metal oxide nanoparticle electrocatalysts through lithium-induced conversion for overall water splitting. Nat. Commun. 2015, 6, 7261–7268.
Dresselhaus, M. S.; Thomas, I. L. Alternative energy technologies. Nature 2001, 414, 332–337.
Ledendecker, M.; Krick Calderon, S.; Papp, C.; Steinruck, H. P.; Antonietti, M.; Shalom, M. The synthesis of nano-structured Ni5P4 films and their use as a non-noble bifunctional electrocatalyst for full water splitting. Angew. Chem., Int. Ed. 2015, 54, 12361–12365.
Jin, H. Y.; Wang, J.; Su, D. F.; Wei, Z. Z.; Pang, Z. F.; Wang, Y. In situ cobalt-cobalt oxide/N-doped carbon hybrids as superior bifunctional electrocatalysts for hydrogen and oxygen evolution. J. Am. Chem. Soc. 2015, 137, 2688–2694.
Tian, J. Q.; Liu, Q.; Cheng, N. Y.; Asiri, A. M.; Sun, X. P. Self-supported Cu3P nanowire arrays as an integrated high-performance three-dimensional cathode for generating hydrogen from water. Angew. Chem., Int. Ed. 2014, 53, 9577–9581.
Wang, J.; Zhong, H. X.; Qin, Y. L.; Zhang, X. B. An efficient three-dimensional oxygen evolution electrode. Angew. Chem., Int. Ed. 2013, 52, 5248–5253.
Li, Y. G.; Wang, H. L.; Xie, L. M.; Liang, Y. Y.; Hong, G. S.; Dai, H. J. MoS2 nanoparticles grown on graphene: An advanced catalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 2011, 133, 7296–7299.
Fan, X. J.; Peng, Z. W.; Ye, R. Q.; Zhou, H. Q.; Guo, X. M3C (M: Fe, Co, Ni) nanocrystals encased in graphene nanoribbons: An active and stable bifunctional electrocatalyst for oxygen reduction and hydrogen evolution reactions. ACS Nano 2015, 9, 7407–7418.
Stern, L. A.; Feng, L. G.; Song, F.; Hu, X. L. Ni2P as a Janus catalyst for water splitting: The oxygen evolution activity of Ni2P nanoparticles. Energy Environ. Sci. 2015, 8, 2347–2351.
Tahir, M.; Mahmood, N.; Zhang, X. X.; Mahmood, T.; Butt, F. K.; Aslam, I.; Tanveer, M.; Idrees, F.; Khalid, S.; Shakir, I. et al. Bifunctional catalysts of Co3O4@GCN tubular nano-structured (TNS) hybrids for oxygen and hydrogen evolution reactions. Nano Res. 2015, 8, 3725–3736.
Tian, J.; Liu, Q.; Asiri, A. M.; Sun, X. P. Self-supported nanoporous cobalt phosphide nanowire arrays: An efficient 3D hydrogen-evolving cathode over the wide range of pH 0–14. J. Am. Chem. Soc. 2014, 136, 7587–7590.
Popczun, E. J.; Read, C. G.; Roske, C. W.; Lewis, N. S.; Schaak, R. E. Highly active electrocatalysis of the hydrogen evolution reaction by cobalt phosphide nanoparticles. Angew. Chem., Int. Ed. 2014, 53, 5427–5430.
Xiao, P.; Sk, M. A.; Thia, L.; Ge, X. M.; Lim, R. J.; Wang, J.-Y.; Lim, K. H.; Wang, X. Molybdenum phosphide as an efficient electrocatalyst for the hydrogen evolution reaction. Energy Environ. Sci. 2014, 7, 2624–2629.
Jiang, P.; Liu, Q.; Liang, Y. H.; Tian, J. Q.; Asiri, A. M.; Sun, X. P. A cost-effective 3D hydrogen evolution cathode with high catalytic activity: FeP nanowire array as the active phase. Angew. Chem., Int. Ed. 2014, 53, 12855–12859.
Pu, Z. H.; Liu, Q.; Tang, C.; Asiri, A. M.; Sun, X. P. Ni2P nanoparticle films supported on a Ti plate as an efficient hydrogen evolution cathode. Nanoscale 2014, 6, 11031–11034.
Popczun, E. J.; McKone, J. R.; Read, C. G.; Biacchi, A. J.; Wiltrout, A. M.; Lewis, N. S.; Schaak, R. E. Nanostructured nickel phosphide as an electrocatalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 2013, 135, 9267–9270.
Li, C. H.; Wang, X.; Peng, Q.; Li, Y. D. Synthesis and characterization of Mn2P2S6 single-crystal nanorods and nanotubes. Inorg. Chem. 2005, 44, 6641–6645.
Liu, P.; Rodriguez, J. A. Catalysts for hydrogen evolution from the [NiFe] hydrogenase to the Ni2P(001) surface: The importance of ensemble effect. J. Am. Chem. Soc. 2005, 127, 14871–14878.
Yang, Y.; Fei, H. L.; Ruan, G. D.; Tour, J. M. Porous cobalt-based thin film as a bifunctional catalyst for hydrogen generation and oxygen generation. Adv. Mater. 2015, 27, 3175–3180.
Zhu, Y. P.; Liu, Y. P.; Ren, T. Z.; Yuan, Z. Y. Self-supported cobalt phosphide mesoporous nanorod arrays: A flexible and bifunctional electrode for highly active electrocatalytic water reduction and oxidation. Adv. Funct. Mater. 2015, 25, 7337–7347.
Wang, D. Y.; Gong, M.; Chou, H. L.; Pan, C. J.; Chen, H. A.; Wu, Y. P.; Lin, M. C.; Guan, M. Y.; Yang, J.; Chen, C. W. et al. Highly active and stable hybrid catalyst of cobalt-doped FeS2 nanosheets–carbon nanotubes for hydrogen evolution reaction. J. Am. Chem. Soc. 2015, 137, 1587–1592.
Friebel, D.; Louie, M. W.; Bajdich, M.; Sanwald, K. E.; Cai, Y.; Wise, A. M.; Cheng, M. J.; Sokaras, D.; Weng, T. C.; Alonso-Mori, R. et al. Identification of highly active Fe sites in (Ni, Fe)OOH for electrocatalytic water splitting. J. Am. Chem. Soc. 2015, 137, 1305–1313.
Gong, M.; Dai, H. J. A mini review of NiFe-based materials as highly active oxygen evolution reaction electrocatalysts. Nano Res. 2015, 8, 23–39.
Wang, H. T.; Tsai, C.; Kong, D. S.; Chan, K. R.; Abild-Pedersen, F.; Nørskov, J. K.; Cui, Y. Transition-metal doped edge sites in vertically aligned MoS2 catalysts for enhanced hydrogen evolution. Nano Res. 2015, 8, 566–575.
Song, L. M.; Zhang, S. J.; Wei, Q. W. Synthesis of highly active and porous NiCoP catalysts via directly thermal treatment of a mechanical mixing of nickel, cobalt salts and sodium hypophosphite. Powder Technol. 2011, 212, 367–371.
Tian, J. Q.; Cheng, N. Y.; Liu, Q.; Xing, W.; Sun, X. P. Cobalt phosphide nanowires: Efficient nanostructures for fluorescence sensing of biomolecules and photocatalytic evolution of dihydrogen from water under visible light. Angew. Chem., Int. Ed. 2015, 54, 5493–5497.
Gu, L.; Wang, Y. W.; Lu, R.; Guan, L.; Peng, X. S.; Sha, J. Anodic electrodeposition of a porous nickel oxide–hydroxide film on passivated nickel foam for supercapacitors. J. Mater. Chem. A 2014, 2, 7161–7164.
McCrory, C. C. L.; Jung, S.; Peters, J. C.; Jaramillo, T. F. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987.
Zhang, H. C.; Li, Y. J.; Zhang, G. X.; Xu, T. H.; Wan, P. B.; Sun, X. M. A metallic CoS2 nanopyramid array grown on 3D carbon fiber paper as an excellent electrocatalyst for hydrogen evolution. J. Mater. Chem. A 2015, 3, 6306–6310.
Zhang, H. C.; Li, Y. J.; Zhang, G. X.; Wan, P. B.; Xu, T. H.; Wu, X. C.; Sun, X. M. Highly crystallized cubic cattierite CoS2 for electrochemically hydrogen evolution over wide pH range from 0 to 14. Electrochim. Acta 2014, 148, 170–174.
Merki, D.; Vrubel, H.; Rovelli, L.; Fierro, S.; Hu, X. L. Fe, Co, and Ni ions promote the catalytic activity of amorphous molybdenum sulfide films for hydrogen evolution. Chem. Sci. 2012, 3, 2515–2525.
Chen, W. F.; Wang, C. H.; Sasaki, K.; Marinkovic, N.; Xu, W.; Muckerman, J. T.; Zhu, Y.; Adzic, R. R. Highly active and durable nano-structured molybdenum carbide electrocatalysts for hydrogen production. Energy Environ. Sci. 2013, 6, 943–951.
Vrubel, H.; Moehl, T.; Grätzel, M.; Hu, X. L. Revealing and accelerating slow electron transport in amorphous molybdenum sulphide particles for hydrogen evolution reaction. Chem. Commun. 2013, 49, 8985–8987.
You, B.; Jiang, N.; Sheng, M. L.; Bhushan, M. W.; Sun, Y. J. Hierarchically porous urchin-like Ni2P superstructures supported on nickel foam as efficient bifunctional electrocatalysts for overall water splitting. ACS Catal. 2016, 6, 714–721.
Yeo, B. S.; Bell, A. T. Enhanced activity of gold-supported cobalt oxide for the electrochemical evolution of oxygen. J. Am. Chem. Soc. 2011, 133, 5587–5593.
Yeo, B. S.; Bell, A. T. In situ Raman study of nickel oxide and gold-supported nickel oxide catalysts for the electrochemical evolution of oxygen. J. Phys. Chem. C 2012, 116, 8394–8400.
Xu, K.; Chen, P. Z.; Li, X. L.; Tong, Y.; Ding, H.; Wu, X. J.; Chu, W. S.; Peng, Z. M.; Wu, C. Z.; Xie, Y. Metallic nickel nitride nanosheets realizing enhanced electrochemical water oxidation. J. Am. Chem. Soc. 2015, 137, 4119–4125.
Masa, J.; Weide, P.; Peeters, D.; Sinev, I.; Xia, W.; Sun, Z. Y.; Somsen, C.; Muhler, M.; Schuhmann, W. Amorphous cobalt boride (Co2B) as a highly efficient nonprecious catalyst for electrochemical water splitting: Oxygen and hydrogen evolution. Adv. Energy Mater. 2016, 6, 1502313.
Tüysüz, H.; Hwang, Y. J.; Khan, S. B.; Asiri, A. M.; Yang, P. D. Mesoporous Co3O4 as an electrocatalyst for water oxidation. Nano Res. 2013, 6, 47–54.
Xia, B. Y.; Yan, Y.; Li, N.; Wu, H. B.; Lou, X. W.; Wang, X. A metal–organic framework-derived bifunctional oxygen electrocatalyst. Nat. Energy 2016, 1, 15006–15013.
Wu, H. B.; Xia, B. Y.; Yu, L.; Yu, X. Y.; Lou, X. W. Porous molybdenum carbide nano-octahedrons synthesized via confined carburization in metal–organic frameworks for efficient hydrogen production. Nat. Commun. 2015, 6, 6512–6519.
Xie, J. F.; Zhang, H.; Li, S.; Wang, R. X.; Sun, X.; Zhou, M.; Zhou, J. F.; Lou, X. W.; Xie, Y. Defect-rich MoS2 ultrathin nanosheets with additional active edge sites for enhanced electrocatalytic hydrogen evolution. Adv. Mater. 2013, 25, 5807–5813.
Zhang, H. C.; Li, Y. J.; Xu, T. H.; Wang, J. B.; Huo, Z. Y.; Wan, P. B.; Sun, X. M. Amorphous Co-doped MoS2 nanosheet coated metallic CoS2 nanocubes as an excellent electrocatalyst for hydrogen evolution. J. Mater. Chem. A 2015, 3, 15020–15023.
Li, Y. J.; Zhang, H. C.; Xu, T. H.; Lu, Z. Y.; Wu, X. C.; Wan, P. B.; Sun, X. M.; Jiang, L. Under-water superaerophobic pine-shaped Pt nanoarray electrode for ultrahigh-performance hydrogen evolution. Adv. Funct. Mater. 2015, 25, 1737–1744.
Lu, Z. Y.; Zhu, W.; Yu, X. Y.; Zhang, H. C.; Li, Y. J.; Sun, X. M.; Wang, X. W.; Wang, H.; Wang, J. M.; Luo, J. et al. Ultrahigh hydrogen evolution performance of under-water “superaerophobic” MoS2 nano-structured electrodes. Adv. Mater. 2014, 26, 2683–2687.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Li, Y., Zhang, H., Jiang, M. et al. Ternary NiCoP nanosheet arrays: An excellent bifunctional catalyst for alkaline overall water splitting. Nano Res. 9, 2251–2259 (2016). https://doi.org/10.1007/s12274-016-1112-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-016-1112-z