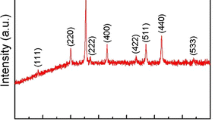
Abstract
Fe3O4 is a promising high-capacity anode material for lithium ion batteries, but challenges including short cycle life and low rate capability hinder its widespread implementation. In this work, a well-defined tubular structure constructed by carbon-coated Fe3O4 has been successfully fabricated with hierarchically porous structure, high surface area, and suitable thickness of carbon layer. Such purposely designed hybrid nanostructures have an enhanced electronic/ionic conductivity, stable electrode/electrolyte interface, and physical buffering effect arising from the nanoscale combination of carbon with Fe3O4, as well as the hollow, aligned and hierarchically porous architectures. When used as an anode material for a lithium-ion half cell, the carbon-coated hierarchical Fe3O4 nanotubes showed excellent cycling performance with a specific capacity of 1,020 mAh·g−1 at 200 mA·g−1 after 150 cycles, a capacity retention of ca. 103%. Even at a higher current density of 1,000 mA·g−1, a capacity of 840 mAh·g−1 is retained after 300 cycles with no capacity loss. In particular, a superior rate capability can be obtained with a stable capacity of 355 mAh·g−1 at 8,000 mA·g−1. The encouraging results indicate that hierarchically tubular hybrid nanostructures can have important implications for the development of high-rate electrodes for future rechargeable lithium ion batteries (LIBs).

Similar content being viewed by others
References
Thackeray, M. M.; Wolverton, C.; Isaacs, E. D. Electrical energy storage for transportation—approaching the limits of, and going beyond, lithium-ion batteries. Energy Environ. Sci. 2012, 5, 7854–7863.
Vu, A.; Qian, Y. Q.; Stein, A. Porous electrode materials for lithium-ion batteries-how to prepare them and what makes them special. Adv. Energy Mater. 2012, 2, 1056–1085.
Reddy, M. V.; Rao, G. V. S.; Chowdari, B. V. R. Metal oxides and oxysalts as anode materials for Li ion batteries. Chem. Rev. 2013, 113, 5364–5457
Jiang, J.; Li, Y. Y.; Liu, J. P.; Huang, X. T. Building one-dimensional oxide nanostructure arrays on conductive metal substrates for lithium-ion battery anodes. Nanoscale 2011, 3, 45–58.
Cao, F. F.; Guo, Y. G.; Wan, L. J. Better lithium-ion batteries with nanocable-like electrode materials. Energy Environ. Sci. 2011, 4, 1634–1642.
Ye, J. F.; Zhang, H. J.; Yang, R.; Li, X. G.; Qi, L. M. Morphology-controlled synthesis of SnO2 nanotubes by using 1D silica mesostructures as sacrificial templates and their applications in lithium-ion batteries. Small 2010, 6, 296–306.
Yoo, J. K.; Kim, J.; Jung, Y. S.; Kang, K. Scalable fabrication of silicon nanotubes and their application to energy storage. Adv. Mater. 2012, 24, 5452–5456.
Jiang, J.; Li, Y. Y.; Liu, J. P.; Huang, X. T.; Yuan, C. Z.; Lou, X. W. Recent advances in metal oxide-based electrode architecture design for electrochemical energy storage. Adv. Mater. 2012, 24, 5166–5180.
Chen, J.; Xu, L.; Li, W.; Gou, X. α-Fe2O3 nanotubes in gas sensor and lithium-ion battery applications. Adv. Mater. 2005, 17, 582–586.
Park, M. H.; Kim, M. G.; Joo, J.; Kim, K.; Kim, J.; Ahn, S.; Cui, Y.; Cho, J. Silicon nanotube battery anodes. Nano Lett. 2009, 9, 3844–3847.
Wang, Z. Y.; Luan, D. Y.; Madhavi, S.; Li, C. M.; Lou, X. W. α-Fe2O3 nanotubes with superior lithium storage capability. Chem. Commun. 2011, 47, 8061–8063.
Zhao, X.; Hayner, C. M.; Kung, M. C.; Kung, H. H. In-plane vacancy-enabled high-power Si-graphene composite electrode for lithium-ion batteries. Adv. Energy Mater. 2011, 1, 1079–1084.
Zhou, J. S.; Song, H. H.; Fu, B. C.; Wu, B.; Chen, X. H. Synthesis and high-rate capability of quadrangular carbon nanotubes with one open end as anode materials for lithium-ion batteries. J. Mater. Chem. 2010, 20, 2794–2800.
Gao, G. X.; Yu, L.; Wu, H. B.; Lou, X. W. Hierarchical tubular structures constructed by carbon-coated α-Fe2O3 nanorods for highly reversible lithium storage. Small 2014, 10, 1741–1745.
Lee, J. E.; Yu, S. H.; Lee, D. J.; Lee, D. C.; Han, S. I.; Sung, Y. E.; Hyeon, T. Facile and economical synthesis of hierarchical carbon-coated magnetite nanocomposite particles and their applications in lithium ion battery anodes. Energy Environ. Sci. 2012, 5, 9528–9533.
Zhang, L.; Wu, H. B.; Lou, X. W. Iron-oxide-based advanced anode materials for lithium-ion batteries. Adv. Energy Mater. 2014, 4, 1300958.
Zhu, J. X.; Yang, D.; Rui, X. H.; Sim, D.; Yu, H.; Hoster, H. E.; Ajayan, P. M.; Yan, Q. Y. Facile preparation of ordered porous graphene-metal oxide@C binder-free electrodes with high Li storage performance. Small 2013, 9, 3390–3397.
He, C. N.; Wu, S.; Zhao, N. Q.; Shi, C. S.; Liu, E. Z.; Li, J. J. Carbon-encapsulated Fe3O4 nanoparticles as a high-rate lithium ion battery anode material. ACS Nano 2013, 7, 4459–4469.
Lin, J.; Raji, A. R. O.; Nan, K. W.; Peng, Z. W.; Yan, Z.; Samuel, E. L. G.; Natelson, D.; Tour, J. M. Iron oxide nanoparticle and graphene nanoribbon composite as an anode material for high-performance Li-ion batteries. Adv. Funct. Mater. 2014, 24, 2044–2048.
Kang, N.; Park, J. H.; Choi, J.; Jin, J.; Chun, J.; Jung, I. G.; Jeong, J.; Park, J. G.; Lee, S. M.; Kim, H. J. et al. Nanoparticulate iron oxide tubes from microporous organic nanotubes as stable anode materials for lithium ion batteries. Angew. Chem. Int. Ed. 2012, 51, 6626–6630.
Han, F.; Li, D.; Li, W. C.; Lei, C.; Sun, Q.; Lu, A. H. Nanoengineered polypyrrole-coated Fe2O3@C multifunctional composites with an improved cycle stability as lithium-ion anodes. Adv. Funct. Mater. 2013, 23, 1692–1700.
Zhang, W. M.; Wu, X. L.; Hu, J. S.; Guo, Y. G.; Wan, L. J. Carbon coated Fe3O4 nanospindles as a superior anode material for lithium-ion batteries. Adv. Funct. Mater. 2008, 18, 3941–3946.
Lee, S. H.; Yu, S. H.; Lee, J. E.; Jin, A.; Lee, D. J.; Lee, N.; Jo, H.; Shin, K.; Ahn, T. Y.; Kim, Y. W. et al. Self-assembled Fe3O4 nanoparticle clusters as high-performance anodes for lithium ion batteries via geometric confinement. Nano Lett. 2013, 13, 4249–4256.
Fang, L.; Shu, Y. Y.; Wang, A. Q.; Zhang, T. Green synthesis and characterization of anisotropic uniform single-crystal α-MoO3 nanostructures. J. Phys. Chem. C 2007, 111, 2401–2408.
Lou, X. W.; Zeng, H. C. Hydrothermal synthesis of α-MoO3 nanorods via acidification of ammonium heptamolybdate tetrahydrate. Chem. Mater. 2002, 14, 4781–4789.
Mao, Y.; Duan, H.; Xu, B.; Zhang, L.; Hu, Y. S.; Zhao, C. C.; Wang, Z. X.; Chen, L. Q.; Yang, Y. S. Lithium storage in nitrogen-rich mesoporous carbon materials. Energy Environ. Sci. 2012, 5, 7950–7955.
Pollak, E.; Salitra, G.; Soffer, A.; Aurbach, D. On the reaction of oxygen with nitrogen-containing and nitrogen-free carbons. Carbon 2006, 44, 3302–3307.
Wei, W.; Yang, S. B.; Zhou, H. X.; Lieberwirth, I.; Feng, X. L.; Müllen, K. 3D graphene foams cross-linked with preencapsulated Fe3O4 nanospheres for enhanced lithium storage. Adv. Mater. 2013, 25, 2909–2914.
Tiwari, S.; Prakash, R.; Choudhary, R. J.; Phase, D. M. Oriented growth of Fe3O4 thin film on crystalline and amorphous substrates by pulsed laser deposition. J. Phys. D: Appl. Phys. 2007, 40, 4943–4947.
Fujii, T.; de Groot, F. M. F.; Sawatzky, G. A.; Voogt, F. C.; Hibma, T.; Okada, K. In situ XPS analysis of various iron oxide films grown by NO2-assisted molecular-beam epitaxy. Phys. Rev. B 1999, 59, 3195–3202.
Wang, R. H.; Xu, C. H.; Du, M.; Sun, J.; Gao, L.; Zhang, P.; Yao, H. L.; Lin, C. C. Solvothermal-induced self-assembly of Fe2O3/GS aerogels for high Li-storage and excellent stability. Small 2014, 10, 2260–2269.
Yan, N.; Zhou, X. H.; Li, Y.; Wang, F.; Zhong, H.; Wang, H.; Chen, Q. W. Fe2O3 nanoparticles wrapped in multi-walled carbon nanotubes with enhanced lithium storage capability. Sci. Rep. 2013, 3, 3392.
Wu, Y.; Wei, Y.; Wang, J. P.; Jiang, K. L.; Fan, S. S. Conformal Fe3O4 sheath on aligned carbon nanotube scaffolds as high-performance anodes for lithium ion batteries. Nano Lett. 2013, 13, 818–823.
Zhou, X. S.; Wan, L. J.; Guo, Y. G. Binding SnO2 nanocrystals in nitrogen-doped graphene sheets as anode materials for lithium-ion batteries. Adv. Mater. 2013, 25, 2152–2157.
Luo, J. S.; Liu, J. L.; Zeng, Z. Y.; Ng, C. F.; Ma, L. J.; Zhang, H.; Lin, J. Y.; Shen, Z. X.; Fan, H. J. Three-dimensional graphene foam supported Fe3O4 lithium battery anodes with long cycle life and high rate capability. Nano Lett. 2013, 13, 6136–6143.
Zhang, G. Q.; Yu, L.; Wu, H. B.; Hoster, H. E.; Lou, X. W. Formation of ZnMn2O4 ball-in-ball hollow microspheres as a high-performance anode for lithium-ion batteries. Adv. Mater. 2012, 24, 4609–4613.
Han, F.; Li, W. C.; Li, M. R.; Lu, A. H. Fabrication of superior-performance SnO2@C composites for lithium-ion anodes using tubular mesoporous carbon with thin carbon walls and high pore volume. J. Mater. Chem. 2012, 22, 9645–9651.
Laruelle, S.; Grugeon, S.; Poizot, P.; Dollé, M.; Dupont, L.; Tarascon, J. M. On the origin of the extra electrochemical capacity displayed by MO/Li cells at low potential. J. Electrochem. Soc. 2002, 149, A627–A634.
Dupont, L.; Laruelle, S.; Grugeon, S.; Dickinson, C.; Zhou, W.; Tarascon, J. M. Mesoporous Cr2O3 as negative electrode in lithium batteries: TEM study of the texture effect on the polymeric layer formation. J. Power Sources 2008, 175, 502–509.
Cherian, C. T.; Sundaramurthy, J.; Kalaivani, M.; Ragupathy, P.; Kumar, P. S.; Thavasi, V.; Reddy, M. V.; Sow, C. H.; Mhaisalkar, S. G.; Ramakrishna, S. et al. Electrospun α-Fe2O3 nanorods as a stable, high capacity anode material for Li-ion batteries. J. Mater. Chem. 2012, 22, 12198–12204.
Wang, P. P.; Sun, H. Y.; Ji, Y. J.; Li, W. H.; Wang, X. Three-dimensional assembly of single-layered MoS2. Adv. Mater. 2014, 26, 964–969.
Xu, S. M.; Hessel, C. M.; Ren, H.; Yu, R. B.; Jin, Q.; Yang, M.; Zhao, H. J.; Wang, D. α-Fe2O3 multi-shelled hollow microspheres for lithium ion battery anodes with superior capacity and charge retention. Energy Environ. Sci. 2014, 7, 632–637.
Chen, Y.; Song, B. H.; Lu, L.; Xue, J. M. Ultra-small Fe3O4 nanoparticle decorated graphene nanosheets with superior cyclic performance and rate capability. Nanoscale 2013, 5, 6797–6803.
Jeong, J. M.; Choi, B. G.; Lee, S. C.; Lee, K. G.; Chang, S. J.; Han, Y. K.; Lee, Y. B.; Lee, H. U.; Kwon, S.; Lee, G. et al. Hierarchical hollow spheres of Fe2O3@polyaniline for lithium ion battery anodes. Adv. Mater. 2013, 25, 6250–6255.
Zhou, G. M.; Wang, D. W.; Yin, L. C.; Li, N.; Li, F.; Cheng, H. M. Oxygen bridges between NiO nanosheets and graphene for improvement of lithium storage. ACS Nano 2012, 6, 3214–3223.
Cao, Z. Y.; Wei, B. Q. High rate capability of hydrogen annealed iron oxide-single walled carbon nanotube hybrid films for lithium-ion batteries. ACS Appl. Mater. Interfaces 2013, 5, 10246–10252.
Mai, L. Q.; Dong, F.; Xu, X.; Luo, Y. Z.; An, Q. Y.; Zhao, Y. L.; Pan, J.; Yang, J. N. Cucumber-like V2O5/poly(3,4-ethylenedioxythiophene)&MnO2 nanowires with enhanced electrochemical cyclability. Nano Lett. 2013, 13, 740–745.
Jia, X. L.; Chen, Z.; Cui, X.; Peng, Y. T.; Wang, X. L.; Wang, G.; Wei, F.; Lu, Y. F. Building robust architectures of carbon and metal oxide nanocrystals toward high-performance anodes for lithium-ion batteries. ACS Nano 2012, 6, 9911–9919.
Lei, C.; Han, F.; Sun, Q.; Li, W. C.; Lu, A. H. Confined nanospace pyrolysis for the fabrication of coaxial Fe3O4@C hollow particles with a penetrated mesochannel as a superior anode for Li-ion batteries. Chem. Eur. J. 2014, 20, 139–145.
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors contributed equally to this work.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Han, F., Ma, L., Sun, Q. et al. Rationally designed carbon-coated Fe3O4 coaxial nanotubes with hierarchical porosity as high-rate anodes for lithium ion batteries. Nano Res. 7, 1706–1717 (2014). https://doi.org/10.1007/s12274-014-0531-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-014-0531-y