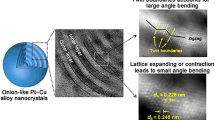
Abstract
Nanocrystal coalescence has attracted paramount attention in nanostructure fabrication in the past decades. Tremendous endeavor and progress have been made in understanding its mechanisms, benefiting from the development of transmission electron microscopy. However, many mechanisms still remain unclear, especially for nanocrystals that lack a permanent dipole moment standing on a solid substrate. Here, we report an in situ coalescence of Pt nanocrystals on an amorphous carbon substrate induced by electron-excitationenhanced van der Waals interactions studied by transmission electron microscopy and first principles calculations. It is found that the electron-beam-induced excitation can significantly enhance the van der Waals interaction between Pt nanocrystals and reduce the binding energy between Pt nanocrystals and the carbon substrate, both of which promote the coalescence. This work extends our understanding of the nanocrystal coalescence observed in a transmission electron microscope and sheds light on a potential pathway toward practical electronbeam-controlled nanofabrication.

Similar content being viewed by others
References
Alivisatos, A. P. Nanocrystals: Building blocks for modern materials design. Endeavour 1997, 21, 56–60.
Sun, Y. G.; Xia, Y. N. Shape-controlled synthesis of gold and silver nanoparticles. Science 2002, 298, 2176–2179.
Rao, C. N. R.; Kulkarni, G. U.; Thomas, P. J.; Edwards, P. P. Size-dependent chemistry: Properties of nanocrystals. Chem. Eur. J. 2002, 8, 28–35.
Zhang, H.; Jin, M. S.; Xiong, Y. J.; Lim, B.; Xia, Y. N. Shape-controlled synthesis of Pd nanocrystals and their catalytic applications. Acc. Chem. Res. 2013, 46, 1783–1794.
Xiao, Q. F.; Weng, D.; Yang, Z. L.; Garay, J.; Zhang, M. J.; Lu, Y. F. Efficient synthesis of PbTe nanoparticle networks. Nano Res. 2010, 3, 685–693.
Penn, R. L.; Banfield, J. F. Morphology development and crystal growth in nanocrystalline aggregates under hydrothermal conditions: Insights from titania. Geochim. Cosmochim. Acta 1999, 63, 1549–1557.
Pacholski, C.; Kornowski, A.; Weller, H. Self-assembly of ZnO: From nanodots to nanorods. Angew. Chem. Int. Edit. 2002, 41, 1188–1191.
van Huis, M. A.; Kunneman, L. T.; Overgaag, K.; Xu, Q.; Pandraud, G.; Zandbergen, H. W.; Vanmaekelbergh, D. Low-temperature nanocrystal unification through rotations and relaxations probed by in situ transmission electron microscopy. Nano Lett. 2008, 8, 3959–3963.
Koga, K.; Takeo, H. In situ observation of coalescence growth of small gold clusters by X-ray diffraction technique. Eur. Phys. J. D 1999, 9, 535–538.
Asoro, M. A.; Kovar, D.; Shao-Horn, Y.; Allard, L. F.; Ferreira, P. J. Coalescence and sintering of Pt nanoparticles: In situ observation by aberration-corrected HAADF STEM. Nanotechnology 2010, 21, 025701.
Liao, H. G.; Cui, L. K.; Whitelam, S.; Zheng, H. M. Real-time imaging of Pt3Fe nanorod growth in solution. Science 2012, 336, 1011–1014.
Courty, A.; Henry, A. I.; Goubet, N.; Pileni, M. P. Large triangular single crystals formed by mild annealing of self-organized silver nanocrystals. Nat. Mater. 2007, 6, 900–907.
Satoh, N.; Hasegawa, H.; Tsujii, K.; Kimura, K. Photoinduced coagulation of Au nanocolloids. J. Phys. Chem. 1994, 98, 2143–2147.
Jin, R. C.; Cao, Y. W.; Mirkin, C. A.; Kelly, K. L.; Schatz, G. C.; Zheng, J. G. Photoinduced conversion of silver nanospheres to nanoprisms. Science 2001, 294, 1901–1903.
Liao, H. G.; Zheng, H. M. Liquid cell transmission electron microscopy study of platinum iron nanocrystal growth and shape evolution. J. Am. Chem. Soc. 2013, 135, 5038–5043.
Giersig, M.; Pastoriza-Santos, I.; Liz-Marzán, L. M. Evidence of an aggregative mechanism during the formation of silver nanowires in N,N-dimethylformamide. J. Mater. Chem. 2004, 14, 607–610.
Dai, Y. Q.; Cobley, C. M.; Zeng, J.; Sun, Y. M.; Xia, Y. N. Synthesis of anatase TiO2 nanocrystals with exposed {001} facets. Nano Lett. 2009, 9, 2455–2459.
Schliehe, C.; Juarez, B. H.; Pelletier, M.; Jander, S.; Greshnykh, D.; Nagel, M.; Meyer, A.; Foerster, S.; Kornowski, A.; Klinke, C. et al. Ultrathin PbS sheets by two-dimensional oriented attachment. Science 2010, 329, 550–553.
Pradhan, N.; Xu, H. F.; Peng, X. G. Colloidal CdSe quantum wires by oriented attachment. Nano Lett. 2006, 6, 720–724.
Smith, D. J.; Petford-Long, A. K.; Wallenberg, L. R.; Bovin, J. O. Dynamic atomic-level rearrangements in small gold particles. Science 1986, 233, 872–875.
Zheng, H. M.; Smith, R. K.; Jun, Y. W.; Kisielowski, C.; Dahmen, U.; Alivisatos, A. P. Observation of single colloidal platinum nanocrystal growth trajectories. Science 2009, 324, 1309–1312.
Li, D. S.; Nielsen, M. H.; Lee, J. R. I.; Frandsen, C.; Banfield, J. F.; De Yoreo, J. J. Direction-specific interactions control crystal growth by oriented attachment. Science 2012, 336, 1014–1018.
Simonsen, S. B.; Chorkendorff, I.; Dahl, S.; Skoglundh, M.; Sehested, J.; Helveg, S. Direct observations of oxygen-induced platinum nanoparticle ripening studied by in situ TEM. J. Am. Chem. Soc. 2010, 132, 7968–7975.
Simonsen, S. B.; Chorkendorff, I.; Dahl, S.; Skoglundh, M.; Sehested, J.; Helveg, S. Ostwald ripening in a Pt/SiO2 model catalyst studied by in situ TEM. J. Catal. 2011, 281, 147–155.
Batson, P. E. Motion of gold atoms on carbon in the aberration-corrected STEM. Microsc. Microanal. 2008, 14, 89–97.
Kurkina, L. I. Static polarizability of excited and charged alkali metal clusters. Phys. Solid State 2001, 43, 792–798.
Tsung, C. K.; Kuhn, J. N.; Huang, W. Y.; Aliaga, C.; Hung, L. I.; Somorjai, G. A.; Yang, P. D. Sub-10 nm platinum nanocrystals with size and shape control: Catalytic study for ethylene and pyrrole hydrogenation. J. Am. Chem. Soc. 2009, 131, 5816–5822.
Koebel, M. M.; Jones, L. C.; Somorjai, G. A. Preparation of size-tunable, highly monodisperse PVP-protected Pt-nanoparticles by seed-mediated growth. J. Nanopart. Res. 2008, 10, 1063–1069.
Malina, D.; Sobczak-Kupiec, A.; Wzorek, Z.; Kowalski, Z. Silver nanoparticles synthesis with different concentrations of polyvinylpyrrolidone. Dig. J. Nanomater. Bios. 2012, 7, 1527–1534.
Edmondson, P. D.; Weber, W. J.; Namavar, F.; Zhang, Y. Determination of the displacement energies of O, Si and Zr under electron beam irradiation. J. Nucl. Mater. 2012, 422, 86–91.
Xu, Q. Y.; Wang, Y.; Wang, Y. G.; Du, X. L.; Xue, Q. K.; Zhang, Z. Polarity determination of ZnO thin films by electron holography. Appl. Phys. Lett. 2004, 84, 2067–2069.
Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799.
Arcidiacono, S.; Bieri, N. R.; Poulikakos, D.; Grigoropoulos, C. P. On the coalescence of gold nanoparticles. Int. J. Multiphase Flow 2004, 30, 979–994.
Batson, P. E.; Reyes-Coronado, A.; Barrera, R. G.; Rivacoba, A.; Echenique, P. M.; Aizpurua, J. Plasmonic nanobilliards: Controlling nanoparticle movement using forces induced by swift electrons. Nano Lett. 2011, 11, 3388–3393.
Batson, P. E.; Reyes-Coronado, A.; Barrera, R. G.; Rivacoba, A.; Echenique, P. M.; Aizpurua, J. Nanoparticle movement: Plasmonic forces and physical constraints. Ultramicroscopy 2012, 123, 50–58.
Polking, M. J.; Urban, J. J.; Milliron, D. J.; Zheng, H. M.; Chan, E.; Caldwell, M. A.; Raoux, S.; Kisielowski, C. F.; Ager, J. W.; Ramesh, R. et al. Size-dependent polar ordering in colloidal GeTe nanocrystals. Nano Lett. 2011, 11, 1147–1152.
Klokkenburg, M.; Houtepen, A. J.; Koole, R.; de Folter, J. W. J.; Erné, B. H.; van Faassen, E.; Vanmaekelbergh, D. Dipolar structures in colloidal dispersions of PbSe and CdSe quantum dots. Nano Lett. 2007, 7, 2931–2936.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Jiang, Y., Wang, Y., Zhang, Y.Y. et al. Direct observation of Pt nanocrystal coalescence induced by electron-excitation-enhanced van der Waals interactions. Nano Res. 7, 308–314 (2014). https://doi.org/10.1007/s12274-013-0396-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-013-0396-5