Abstract
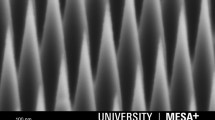
We describe the fabrication of arrays of oxide nanotubes using a combination of bottom up and top down nanofabrication. The nanotubes are made from epitaxially grown semiconductor nanowires that are covered with an oxide layer using atomic layer deposition. The tips of the oxide-covered nanowires are removed by argon sputtering and the exposed semiconductor core is then selectively etched, leaving a hollow oxide tube. We show that it is possible to create fluidic connections to the nanotubes by a combination of electron beam lithography to precisely define the nanotube positions and controlled wet under-etching. DNA transport is demonstrated in the microchannel. Cells were successfully cultured on the nanotube arrays, demonstrating compatibility with cell-biological applications. Our device opens up the possibility of injecting molecules into cells with both spatial and temporal control.

Similar content being viewed by others
References
Prinz, C.; Hallstrom, W.; Martensson, T.; Samuelson, L.; Montelius, L.; Kanje, M. Axonal guidance on patterned free-standing nanowire surfaces. Nanotechnology 2008, 19, 345101.
Hallstrom, W.; Prinz, C. N.; Suyatin, D.; Samuelson, L.; Montelius, L.; Kanje, M. Rectifying and sorting of regenerating axons by free-standing nanowire patterns: A highway for nerve fibers. Langmuir 2009, 25, 4343–4346.
Hallstrom, W.; Lexholm, M.; Suyatin, D. B.; Hammarin, G.; Hessman, D.; Samuelson, L.; Montelius, L.; Kanje, M.; Prinz, C. N. Fifteen-piconewton force detection from neural growth cones using nanowire arrays. Nano Lett. 2010, 10, 782–787.
Zheng, G. F.; Patolsky, F.; Cui, Y.; Wang, W. U.; Lieber, C. M. Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nat. Biotechnol. 2005, 23, 1294–1301.
Dimaki, M.; Vazquez, P.; Olsen, M. H.; Sasso, L.; Rodriguez-Trujillo, R.; Vedarethinam, I.; Svendsen, W. E. Fabrication and characterization of 3d micro- and nanoelectrodes for neuron recordings. Sensors 2010, 10, 10339–10355.
Linsmeier, C. E.; Prinz, C. N.; Pettersson, L. M. E.; Caroff, P.; Samuelson, L.; Schouenborg, J.; Montelius, L.; Danielsen, N. Nanowire biocompatibility in the brain-Looking for a needle in a 3d stack. Nano Lett. 2009, 9, 4184–4190.
Hallstrom, W.; Martensson, T.; Prinz, C.; Gustavsson, P.; Montelius, L.; Samuelson, L.; Kanje, M. Gallium phosphide nanowires as a substrate for cultured neurons. Nano Lett. 2007, 7, 2960–2965.
Berthing, T.; Bonde, S.; Sorensen, C. B.; Utko, P.; Nygard, J.; Martinez, K. L. Intact mammalian cell function on semiconductor nanowire arrays: New perspectives for cell-based biosensing. Small 2011, 7, 640–647.
Park, S.; Kim, Y. -S.; Kim, W. B.; Jon, S. Carbon nanosyringe array as a platform for intracellular delivery. Nano Lett. 2009, 9, 1325–1329.
McKnight, T. E.; Melechko, A. V.; Griffin, G. D.; Guillorn, M. A.; Merkulov, V. I.; Serna, F.; Hensley, D. K.; Doktycz, M. J.; Lowndes, D. H.; Simpson, M. L. Intracellular integration of synthetic nanostructures with viable cells for controlled biochemical manipulation. Nanotechnology 2003, 14, 551–556.
Shalek, A. K.; Robinsson, J. T.; Karp, E. S.; Lee, J. S.; Ahn, D. -R.; Yoon, M. -H.; Sutton, A.; Jorgolli, M.; Gertner, R. S.; Gujral, T. S., et al. Vertical silicon nanowires as a universal platform for delivering biomolecules into living cells. P. Natl. Acad. Sci. USA 2010, 107, 1870–1875.
Kim, W.; Ng, J. K.; Kunitake, M. E.; Conklin, B. R.; Yang, P. D. Interfacing silicon nanowires with mammalian cells. J. Am. Chem. Soc. 2007, 129, 7228–7229.
Meister, A.; Gabi, M.; Behr, P.; Studer, P.; Voros, J.; Niedermann, P.; Bitterli, J.; Polesel-Maris, J.; Liley, M.; Heinzelmann, H., et al. FluidFM: Combining atomic force microscopy and nanofluidics in a universal liquid delivery system for single cell applications and beyond. Nano Lett. 2009, 9, 2501–2507.
Chen, X.; Kis, A.; Zettl, A.; Bertozzi, C. R. A cell nanoinjector based on carbon nanotubes P. Natl. Acad. Sci. USA 2007, 104, 8218–8222.
Singhal, R.; Orynbayeva, Z.; Sundaram, R. V. K.; Niu, J. J.; Bhattacharyya, S.; Vitol, E. A.; Schrlau, M. G.; Papazoglou, E. S.; Friedman, G.; Gogotsi, Y. Multifunctional carbon-nanotube cellular endoscopes. Nat. Nanotechnol. 2011, 6, 57–64.
Vakarelski, I. U.; Brown, S. C.; Higashitani, K.; Moudgil, B. M. Penetration of living cell membranes with fortified carbon nanotube tips. Langmuir 2007, 23, 10893–10896.
Kobayashi, N.; Rivas-Carillo, J. D.; Soto-Gutierrez, A.; Fukazawa, T.; Chen, Y.; Navarro-Alvarez, N.; Tanaka, N. Gene delivery to embryonic stem cells. Birth Defects Res. C 2005, 75, 10–18.
Karra, D.; Dahm, R. Transfection techniques for neuronal cells. J. Neurosci. 2010, 30, 6171–6177.
Zhang, Y.; Yu, L. C. Microinjection as a tool of mechanical delivery. Curr Opin Biotechnol. 2008, 19, 506–510.
Zhang, Y.; Yu, L. C. Single-cell microinjection technology in cell biology. BioEssays 2008, 30, 606–610.
McAllister, D. V.; Allen, M. G.; Prausnitz, M. R. Microfabricated microneedles for gene and drug delivery. Annu. Rev. Biomed. Eng. 2000, 2, 289–313.
Adamo, A.; Jensen, K. F. Microfluidic based single cell microinjection. Lab Chip 2008, 8, 1258–1261.
Matsuoka, H.; Komazaki, T.; Mukai, Y.; Shibusawa, M.; Akane, H.; Chaki, A.; Uetake, N.; Saito, M. High throughput easy microinjection with a single-cell manipulation supporting robot. J. Biotechnol. 2005, 116, 185–194.
Schrlau, M. G.; Falls, E. M.; Ziober, B. L.; Bau, H. H. Carbon nanopipettes for cell probes and intracellular injection. Nanotechnology 2008, 19, 015101.
Lee, S.; An, R.; Hunt, A. J. Liquid glass electrodes for nanofluidics. Nat. Nanotechnol. 2010, 5, 412–416.
Kim, B. M.; Murray, T.; Bau, H. H. The fabrication of integrated carbon pipes with sub-micron diameters. Nanotechnology 2005, 16, 1317–1320.
Han, S. W.; Nakamura, C.; Kotobuki, N.; Obataya, I.; Ohgushi, H.; Nagamune, T.; Miyake, J. High-efficiency DNA injection into a single human mesenchymal stem cell using a nanoneedle and atomic force microscopy. Nanomed. Nanotechnol. 2008, 4, 215–225.
Kouklin, N. A.; Kim, W. E.; Lazareck, A. D.; Xu, J. M. Carbon nanotube probes for single-cell experimentation and assays. Appl. Phys. Lett. 2005, 87, 173901.
Skold, N.; Hallstrom, W.; Persson, H.; Montelius, L.; Kanje, M.; Samuelson, L.; Prinz, C. N.; Tegenfeldt, J. O. Nanofluidics in hollow nanowires. Nanotechnology. 2010, 21, 155301.
Messing, M. E.; Hillerich, K.; Bolinsson, J.; Storm, K.; Johansson, J.; Dick, K. A.; Deppert, K. A comparative study of the effect of gold seed particle preparation method on nanowire growth. Nano Res. 2010, 3, 506–519.
Suyatin, D. B.; Hallstrom, W.; Samuelson, L.; Montelius, L.; Prinz, C. N.; Kanje, M. Gallium phosphide nanowire arrays and their possible application in cellular force investigations. J. Vac. Sci. Technol. B 2009, 27, 3092–3094.
Chang, K. L.; Lee, C. K.; Hsu, J. W.; Hsieh, H. F.; H.C., S. The etching behavior of n-gap in aqua regia solutions. J. Appl. Electrochem. 2005, 35, 77–84.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Supplementary material, approximately 4.46 MB
Rights and permissions
About this article
Cite this article
Persson, H., Beech, J.P., Samuelson, L. et al. Vertical oxide nanotubes connected by subsurface microchannels. Nano Res. 5, 190–198 (2012). https://doi.org/10.1007/s12274-012-0199-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-012-0199-0