Abstract
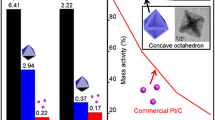
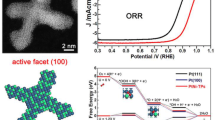
Platinum (Pt) is an outstanding catalyst for many important industrial products. Because of its high cost and scarce reserves, it is very important to improve the performance of Pt catalysts. As the metal nanocrystals (NCs) with high-index surfaces usually show very good catalytic activity because of their high density of atomic steps and kinks, the preparation of Pt NCs with high-index facets has become a very important and hot research topic recently. In this article, we report a facile synthesis of high-yield Pt NCs with a series of {hkk} high-index facets including {211} and {411} via a solvothermal method using Pt(II) acetylacetonate as the Pt source, 1-octylamine as the solvent and capping agent, and formaldehyde as an additional surface structure regulator. Multipod Pt NCs with dominant {211} side surfaces were produced without formaldehyde, while concave Pt NCs with dominant {411} surfaces formed under the influence of formaldehyde. By analyzing the products by IR spectroscopy, we found the presence of CO on the surface of concave Pt NCs with {411} surfaces prepared from the solution containing formaldehyde. It was concluded that amine mainly stabilized the monoatomic step edges, resulting in the {211} exposed surface; with addition of formaldehyde, it decomposed into CO, leading to the formation of {411} surfaces by the additional adsorption of the CO on the {100} terraces. In addition, it was found that the as-prepared Pt NCs with high-index {211} and {411} surfaces exhibited much better catalytic activity in the electro-oxidation of ethanol than a commercial Pt/C catalyst or Pt nanocubes with low-index {100} surfaces, and the catalytic activities of Pt crystal facets decreased in the sequence {411}>{211}>{100}.

Similar content being viewed by others
References
Peng, Z. M.; Yang, H. Designer platinum nanoparticles: Control of shape, composition in alloy, nanostructure and electrocatalytic property. Nano Today 2009, 4, 143–164.
Chen, J. Y.; Lim, B.; Lee, E. P.; Xia, Y. N. Shape-controlled synthesis of platinum nanocrystals for catalytic and electrocatalytic applications. Nano Today 2009, 4, 81–95.
Chen, A. C.; Holt-Hindle, P. Platinum-based nanostructured materials: Synthesis, properties, and applications. Chem. Rev. 2010, 110, 3767–3804.
Cheong, S. S.; Watt, J. D.; Tilley, R. D. Shape control of platinum and palladium nanoparticles for catalysis. Nanoscale 2010, 2, 2045–2053.
Pimentel, G. C. Opportunities in Chemistry; National Academy Press: Washington, D. C., 1985; pp 193–265.
Tian, N.; Zhou, Z. Y.; Sun, S. G. Platinum metal catalysts of high-index surfaces: From single-crystal planes to electrochemically shape-controlled nanoparticles. J. Phys. Chem. C 2008, 112, 19801–19817.
Jiang, Z. Y.; Kuang, Q.; Xie, Z. X.; Zheng, L. S. Syntheses and properties of micro/nanostructured crystallites with high-energy surfaces. Adv. Funct. Mater. 2010, 20, 3634–3645.
Zhou, Z. Y.; Tian, N.; Li, J. T.; Broadwell, I.; Sun, S. G. Nanomaterials of high surface energy with exceptional properties in catalysis and energy storage. Chem. Soc. Rev. 2011, 40, 4167–4185.
Teranishi, T.; Kurita, R.; Miyake, M. Shape control of Pt nanoparticles. J. Inorg. Organomet. Polym. 2000, 10, 145–156.
Herricks, T.; Chen, J. Y.; Xia, Y. N. Polyol synthesis of platinum nanoparticles: Control of morphology with sodium nitrate. Nano Lett. 2004, 4, 2367–2371.
Elechiguerra, J. L.; Larios-Lopez, L.; Jose-Yacaman, M. Controlled synthesis of platinum submicron and nanometric particles with novel shapes. Appl. Phys. A 2006, 84, 11–19.
Ren, J. T.; Tilley, R. D. Preparation, self-assembly, and mechanistic study of highly monodispersed nanocubes. J. Am. Chem. Soc. 2007, 129, 3287–3291.
Maksimuk, S.; Teng, X. W.; Yang, H. Roles of twin defects in the formation of platinum multipod nanocrystals. J. Phys. Chem. C 2007, 111, 14312–14319.
Demortière, A.; Launois, P.; Goubet, N.; Albouy, P. A.; Petit, C. Shape-controlled platinum nanocubes and their assembly into two-dimensional and three-dimensional superlattices. J. Phys. Chem. B 2008, 112, 14583–14592.
Ren, J. T.; Tilley, R. D. Shape-controlled growth of platinum nanoparticles. Small 2007, 3, 1508–1512.
Lim, S. I.; Ojea-Jiménez, I.; Varon, M.; Casals, E.; Arbiol, J.; Puntes, V. Synthesis of platinum cubes, polypods, cuboctahedrons, and raspberries assisted by cobalt nanocrystals. Nano Lett. 2010, 10, 964–973.
Tian, N.; Zhou, Z. Y.; Sun, S. G.; Ding, Y.; Wang, Z. L. Synthesis of tetrahexahedral platinum nanocrystals with high-index facets and high electro-oxidation activity. Science 2007, 316, 732–735.
Zhou, Z. Y.; Huang, Z. Z.; Chen, D. J.; Wang, Q.; Tian, N.; Sun, S. G. High-index faceted platinum nanocrystals supported on carbon black as highly efficient catalysts for ethanol electrooxidation. Angew. Chem. Int. Ed. 2010, 49, 411–414.
Ma, Y. Y.; Kuang, Q.; Jiang, Z. Y.; Xie, Z. X.; Huang, R. B.; Zheng, L. S. Synthesis of trisoctahedral gold nanocrystals with exposed high-index facets by a facile chemical method Angew. Chem. Int. Ed. 2008, 47, 8901–8904.
Ming, T.; Feng, W.; Tang, Q.; Wang, F.; Sun, L. D.; Wang, J. F.; Yan, C. H. Growth of tetrahexahedral gold nanocrystals with high-index facets. J. Am. Chem. Soc. 2009, 131, 16350–16351.
Zhang, J.; Langille, M. R.; Personick, M. L.; Zhang, K.; Li, S. Y.; Mirkin, C. A. Concave cubic gold nanocrystals with high-index facets J. Am. Chem. Soc. 2010, 132, 14012–14014.
Yu, Y.; Zhang, Q. B.; Lu, X. M.; Lee, J. Y. Seed-mediated synthesis of monodisperse concave trisoctahedral gold nanocrystals with controllable sizes J. Phys. Chem. C 2010, 114, 11119–11126.
Li, J.; Wang, L. H.; Liu, L.; Guo, L.; Han, X. D.; Zhang, Z. Synthesis of tetrahexahedral Au nanocrystals with exposed high-index surfaces. Chem. Commun. 2010, 46, 5109–5111.
Lu, C. L.; Prasad, K. S.; Wu, H. L.; Ho, J. A. A.; Huang, M. H. Au nanocube-directed fabrication of Au-Pd core-shell nanocrystals with tetrahexahedral, concave octahedral, and octahedral structures and their electrocatalytic activity. J. Am. Chem. Soc. 2010, 132, 14546–14553.
Yu, Y.; Zhang, Q. B.; Liu, B.; Lee, J. Y. Synthesis of nanocrystals with variable high-index Pd facets through the controlled heteroepitaxial growth of trisoctahedral Au templates. J. Am. Chem. Soc. 2010, 132, 18258–18265.
Wang, F.; Li, C. H.; Sun, L. D.; Wu, H. S.; Ming, T.; Wang, J. F.; Yu, J. C.; Yan, C. H. Heteroepitaxial growth of high-index-faceted palladium nanoshells and their catalytic performance. J. Am. Chem. Soc. 2011, 133, 1106–1111.
Jiang, Q. N.; Jiang, Z. Y.; Zhang, L.; Lin, H. X.; Yang, N.; Li, H.; Liu, D. Y.; Xie, Z. X.; Tian, Z. Q. Synthesis and high electrocatalytic performance of hexagram shaped gold particles having an open surface structure with kinks. Nano Res. 2011, 4, 612–622.
Zhang, J. W.; Zhang, L.; Xie, S. F.; Kuang, Q.; Han, X. G.; Xie, Z. X.; Zheng, L. S. Synthesis of concave palladium nanocubes with high-index surfaces and high electrocatalytic activities. Chem. Eur. J. 2011, 17, 9915–9919.
Niu, W. X.; Xu, G. B. Crystallographic control of noble metal nanocrystals. Nano Today 2011, 6, 265–285.
Yu, T.; Kim, D. Y.; Zhang, H.; Xia, Y. Platinum concave nanocubes with high-index facets and their enhanced activity for oxygen reduction reaction. Angew. Chem. Int. Ed. 2011, 50, 2773–2777.
Huang, X. Q.; Zhao, Z. P.; Fan, J. M.; Tan, Y. M.; Zheng, N. F. Amine-assisted synthesis of concave polyhedral platinum nanocrystals having {411} high-index facets. J. Am. Chem. Soc. 2011, 133, 4718–4721.
Kim, C.; Lee, H. Shape effect of Pt nanocrystals on electrocatalytic hydrogenation. Catal. Commun. 2009, 11, 7–10.
Nakamura, M.; Hanioka, Y.; Ouchida, W.; Yamada, M. Hoshi, N. Estimation of surface structure and carbon monoxide oxidation site of shape-controlled Pt nanoparticles. ChemPhysChem 2009, 10, 2719–2724.
Sun, S. G.; Zhou, Z. Y. Surface processes and kinetics of CO2 reduction on Pt(100) electrodes of different surface structure in sulfuric acid solutions. Phys. Chem. Chem. Phys. 2001, 3, 3277–3283.
Wu, B. H.; Zheng, N. F.; Fu, G. Small molecules control the formation of Pt nanocrystals: A key role of carbon monoxide in the synthesis of Pt nanocubes. Chem. Commun. 2011, 47, 1039–1041.
Wang, Z. L.; Ahmad, T. S.; El-Sayed, M. A. Steps, ledges and kinks on the surfaces of platinum nanoparticles of different shapes. Surf. Sci. 1997, 380, 302–310.
Hitmi, H.; Belgsir, E. M.; Leger, J. M.; Lamy, C.; Lezna, R. O. A kinetic analysis of the electro-oxidation of ethanol at a platinum electrode in acid medium. Electrochim. Acta 1994, 39, 407–415.
Abd-el-latif, A. A.; Mostafa, E.; Huxter, S.; Attard, G.; Baltruschat, H. Electrooxidation of ethanol at polycrystalline and platinum stepped single crystals: A study by differential electrochemical mass spectrometry. Electrochim. Acta 2010, 55, 7951–7960.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, L., Chen, D., Jiang, Z. et al. Facile syntheses and enhanced electrocatalytic activities of Pt nanocrystals with {hkk} high-index surfaces. Nano Res. 5, 181–189 (2012). https://doi.org/10.1007/s12274-012-0198-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-012-0198-1