Abstract
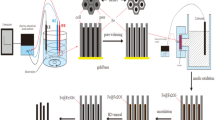
We report the synthesis of one-dimensional (1-D) magnetic Fe2P nanowires and Fe2P@C core@shell nanocables by the reactions of triphenylphosphine (PPh3) with Fe powder (particles) and ferrocene (Fe(5H5)2), respectively, in vacuum-sealed ampoules at 380–400 °C. The synthesis is based on chemical conversion of micrometer or nanometer sized Fe particles into Fe2P via the extraction of phosphorus from liquid PPh3 at elevated temperatures. In order to control product diameters, a convenient sudden-temperature-rise strategy is employed, by means of which diameter-uniform Fe2P@C nanocables are prepared from the molecular precursor Fe(C5H5)2. In contrast, this strategy gives no obvious control over the diameters of the Fe2P nanowires obtained using elemental Fe as iron precursor. The formation of 1-D Fe2P nanostructures is ascribed to the cooperative effects of the kinetically induced anisotropic growth and the intrinsically anisotropic nature of hexagonal Fe2P crystals. The resulting Fe2P nanowires and Fe2P@C nanocables display interesting ferromagnetic-paramagnetic transition behaviors with blocking temperatures of 230 and 268 K, respectively, significantly higher than the ferromagnetic transition temperature of bulk Fe2P (T C = 217 K).

Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Xia, Y. N.; Yang, P. D.; Sun, Y. G.; Wu, Y. Y.; Mayers, B.; Gates, B.; Yin, Y. D.; Kim, F.; Yan, H. Q. One-dimensional nanostructures: Synthesis, characterization, and applications. Adv. Mater. 2003, 15, 353–389.
Law, M.; Goldberger, J.; Yang, P. D. Semiconductor nanowires and nanotubes. Ann. Rev. Mater. Res. 2004, 34, 83–122.
Li, Y.; Qian, F.; Xiang, J.; Lieber, C. M. Nanowire electronic and optoelectronic devices. Mater. Today 2006, 9, 18–27.
Wang, Z. L.; Song, J. H. Piezoelectric nanogenerators based on zinc oxide nanowire arrays. Science 2006, 312, 242–246.
Xu, C.; Wang, X. D.; Wang, Z. L. Nanowire structured hybrid cell for concurrently scavenging solar and mechanical energies. J. Am. Chem. Soc. 2009, 131, 5866–5872.
Aronsson, B.; Lundstrom, T.; Rundquist, S. Borides, Silicides, and Phosphides: A Critical Review of Their Preparation, Properties, and Crystal Chemistry; Ballantyne and Co.: Spottiswoode, U.K., 1965.
Oyama, S. T. Novel catalysts for advanced hydroprocessing: Transition metal phosphides. J. Catal. 2003, 216, 343–352.
Gschneidner Jr. K. A.; Pecharsky, V. K.; Tsokol, A. O. Recent developments in magnetocaloric materials. Rep. Prog. Phys. 2005, 68, 1479–1539.
Gillot, F.; Boyanov, S.; Dupont, L.; Doublet, M. L.; Morcrette, M.; Monconduit, L.; Tarascon, J. M. Electrochemical reactivity and design of NiP2 negative electrodes for secondary Li-ion batteries. Chem. Mater. 2005, 17, 6327–6337.
Boyanov, S.; Bernardi, J.; Gillot, F.; Dupont, L.; Womes, M.; Tarascon, J. M.; Monconduit, L.; Doublet, M. L. FeP: Another attractive anode for the Li-ion battery enlisting a reversible two-step insertion/conversion process. Chem. Mater. 2006, 18, 3531–3538.
Xia, Y. N.; Xiong, Y. J.; Lim, B.; Skrabalak, S. E. Shape-controlled synthesis of metal nanocrystals: Simple chemistry meets complex physics? Angew. Chem. Int. Ed. 2009, 48, 60–103.
Sun, S. H.; Murray, C. B.; Weller, D.; Folks, L.; Moser, A. Monodisperse FePt nanoparticles and ferromagnetic FePt nanocrystal superlattices. Science 2000, 287, 1989–1992.
Comini, E.; Baratto, C.; Faglia, G.; Ferroni, M.; Vomiero, A.; Sberveglieri, G. Quasi-one-dimensional metal oxide semiconductors: Preparation, characterization and application as chemical sensors. Prog. Mater. Sci. 2009, 54, 1–67.
Jun, Y.; Choi, J.; Cheon, J. Shape control of semiconductor and metal oxide nanocrystals through nonhydrolytic colloidal routes. Angew. Chem. Int. Ed. 2006, 45, 3414–3439.
Peng, X. G.; Wickham, J.; Alivisatos, A. P. Kinetics of II- VI and III-V colloidal semiconductor nanocrystal growth: “Focusing” of size distributions. J. Am. Chem. Soc. 1998, 120, 5343–5344.
Wang, X.; Zhuang, J.; Peng, Q.; Li, Y. D. A general strategy for nanocrystal synthesis. Nature 2005, 437, 121–124.
Homma, Y.; Liu, H. P.; Takagi, D.; Kobayashi, Y. Single-walled carbon nanotube growth with non-iron-group “catalysts” by chemical vapor deposition. Nano Res. 2009, 2, 793–799.
Kodambaka, S.; Tersoff, J.; Reuter, M. C.; Ross, F. M. Germanium nanowire growth below the eutectic temperature. Science 2007, 316, 729–732.
Wang, F.; Buhro, W. E. Determination of the rod.wire transition length in colloidal indium phosphide quantum rods. J. Am. Chem. Soc. 2007, 129, 14381–14387.
Fanfair, D. D.; Korgel, B. A. Bismuth nanocrystal-seeded III-V semiconductor nanowire synthesis. Cryst. Growth Des. 2005, 5, 1971–1976.
Brock, S. L.; Perera, S. C.; Stamm, K. L. Chemical routes for production of transition metal phosphides on the nanoscale: Implications for advanced magnetic and catalytic materials. Chem. Eur. J. 2004, 10, 3364–3371.
Park, J.; Koo, B.; Yoon, K. Y.; Hwang, Y.; Kang, M.; Park, J. G.; Hyeon, T. Generalized synthesis of metal phosphide nanorods via thermal decomposition of continuously delivered metal-phosphine complexes using a syringe pump. J. Am. Chem. Soc. 2005, 127, 8433–8440.
Park, J.; Koo, B.; Hwang, Y.; Bae, C.; An, K.; Park, J. G.; Park, H. M.; Hyeon, T. Novel synthesis of magnetic Fe2P nanorods from thermal decomposition of continuously delivered precursors using a syringe pump. Angew. Chem., Int. Ed. 2004, 43, 2282–2285.
Chen, J. H.; Taib, M. F.; Chi, K. M. Catalytic synthesis, characterization and magnetic properties of iron phosphide nanowires. J. Mater. Chem. 2004, 14, 296–298.
Qian, C.; Kim, F.; Ma, L.; Tsui, F.; Yang, P. D.; Liu, J. Solution-phase synthesis of single-crystalline iron phosphide nanorods/nanowires. J. Am. Chem. Soc. 2004, 126, 1195–1198.
Gregg, K. A.; Perera, S. C.; Lawes, G.; Shinozaki, S.; Brock, S. L. Controlled synthesis of MnP nanorods: Effect of shape anisotropy on magnetization. Chem. Mater. 2006, 18, 879–886.
Li, Y.; Malik, M. A.; O’Brien, P. Synthesis of single-crystalline CoP nanowires by a one-pot metal-organic route. J. Am. Chem. Soc. 2005, 127, 16020–16021.
Kelly, A. T.; Rusakova, I.; Ould-Ely, T.; Hofmann, C.; Lüttge, A.; Whitmire, K. H. Iron phosphide nanostructures produced from a single-source organometallic precursor: Nanorods, bundles, crosses, and spherulites. Nano Lett. 2007, 7, 2920–2925.
Lukehart, C. M.; Milne, S. B.; Stock, S. R. Formation of crystalline nanoclusters of Fe2P, RuP, Co2P, Rh2P, Ni2P, Pd5P2, or PtP2 in a silica xerogel matrix from single-source molecular precursors. Chem. Mater. 1998, 10, 903–908.
Xie, Y.; Su, H. L.; Qian, X. F.; Liu, X. M.; Qian, Y. T. A mild one-step solvothermal route to metal phosphides (metal = Co, Ni, Cu). J. Solid State Chem. 2000, 149, 88–91.
Luo, F.; Su, H. L.; Song, W.; Wang, Z. M.; Yan, Z. G.; Yan, C. H. Magnetic and magnetotransport properties of Fe2P nanocrystallites via a solvothermal route. J. Mater. Chem. 2004, 14, 111–115.
Perera, S. C.; Tsoi, G.; Wenger, L. E.; Brock, S. L. Synthesis of MnP nanocrystals by treatment of metal carbonyl complexes with phosphines: A new, versatile route to nanoscale transition metal phosphides. J. Am. Chem. Soc. 2003, 125, 13960–13961.
Stamm, K. L.; Garno, J. C.; Liu, G. Y.; Brock, S. L. A general methodology for the synthesis of transition metal pnictide nanoparticles from pnictate precursors and its application to iron-phosphorus phases. J. Am. Chem. Soc. 2003, 125, 4038–4039.
Chiang, R. K.; Chiang, R. T. Formation of hollow Ni2P nanoparticles based on the nanoscale Kirkendall effect. Inorg. Chem. 2007, 46, 369–371.
Henkes, A. E.; Vasquez, Y.; Schaak, R. E. Converting metals into phosphides: A general strategy for the synthesis of metal phosphide nanocrystals. J. Am. Chem. Soc. 2007, 129, 1896–1897.
Vasquez, Y.; Henkes, A. E.; Bauer, J. C.; Schaak, R. E. Nanocrystal conversion chemistry: A unified and materialsgeneral strategy for the template-based synthesis of nanocrystalline solids. J. Solid State Chem. 2008, 181, 1509–1523.
Wang, J. L.; Yang, Q.; Zhang, Z. D.; Sun, S. Phase-controlled synthesis of transition metal phosphide nanowires via new Ullmann-type reactions. Chem. Eur. J. 2010, accepted.
Wang, J. L.; Yang, Q.; Zhang, Z. D. Selective synthesis of magnetic Fe2P/C and FeP/C core/shell nanocables. J. Phys. Chem. Lett. 2010, 1, 102–106.
Kumar, S.; Nann, T. Shape control of II-VI semiconductor nanomaterials. Small 2006, 2, 316–329.
Robb, D. T.; Privman, V. Model of nanocrystal formation in solution by burst nucleation and diffusional growth. Langmuir 2008, 24, 26–35.
Predel, B. Fe-P (Iron-Phosphorus). Madelung, O., Ed.; Springermaterials—The Landolt-Börnstein Database. http://www.springermaterials.com, DOI: 10.1007/10474837_1325.
Fujii, H.; Uwatoko, Y.; Motoya, K.; Ito, Y.; Okamoto, T. Neutron scattering investigation of itinerant electron system Fe2P. J. Phys. Soc. Jpn. 1988, 57, 2143–2153.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published with open access at Springerlink.com
Electronic supplementary material
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Wang, J., Yang, Q., Zhou, J. et al. Magnetic Fe2P nanowires and Fe2P@C core@shell nanocables. Nano Res. 3, 211–221 (2010). https://doi.org/10.1007/s12274-010-1024-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-010-1024-2