Abstract
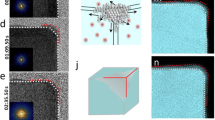
This paper presents a systematic study of the growth mechanism for Pd nanobars synthesized by reducing Na2PdCl4 with L-ascorbic acid in an aqueous solution in the presence of bromide ions as a capping agent. Transmission electron microscopy (TEM) and high-resolution TEM analyses revealed that the growth at early stages of the synthesis was dominated by particle coalescence, followed by shape focusing via recrystallization and further growth via atomic addition. We also investigated the detailed surface structure of the nanobars using aberration-corrected scanning TEM and found that the exposed {100} surfaces contained several types of defects such as an adatom island, a vacancy pit, and atomic steps. Upon thermal annealing, the nanobars evolved into a more thermodynamically favored shape with enhanced truncation at the corners.

Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Skrabalak, S. E.; Chen, J.; Sun, Y.; Lu, X.; Au, L.; Cobley, C. M.; Xia, Y. Gold nanocages: Synthesis, properties, and applications. Acc. Chem. Res. 2008, 41, 1587–1595.
Xia, Y.; Xiong, Y.; Lim, B.; Skrabalak, S. E. Shape-controlled synthesis of metal nanocrystals: Simple chemistry meets complex physics? Angew. Chem. Int. Ed. 2009, 48, 60–103.
Peng, Z.; Yang, H. Designer platinum nanoparticles: Control of shape, composition in alloy, nanostructure and electrocatalytic property. Nano Today 2009, 4, 143–164.
Tian, N.; Zhou, Z. -Y.; Sun, S. -G.; Ding, Y.; Wang, Z. L. Synthesis of tetrahexahedral platinum nanocrystals with high-index facets and high electro-oxidation activity. Science 2007, 316, 732–735.
Bratlie, K. M.; Lee, H.; Komvopoulos, K.; Yang, P.; Somorjai, G. A. Platinum nanoparticle shape effects on benzene hydrogenation selectivity. Nano Lett. 2007, 7, 3097–3101.
Lim, B.; Lu, X.; Jiang, M.; Camargo, P. H. C.; Cho, E. C.; Lee, E. P.; Xia, Y. Facile synthesis of highly faceted multioctahedral Pt nanocrystals through controlled overgrowth. Nano Lett. 2008, 8, 4043–4047.
Wang, C.; Daimon, H.; Onodera, T.; Koda, T.; Sun, S. A general approach to the size- and shape-controlled synthesis of platinum nanoparticles and their catalytic reduction of oxygen. Angew. Chem. Int. Ed. 2008, 47, 3588–3591.
Lim, B.; Jiang, M.; Camargo, P. H. C.; Cho, E. C.; Tao, J.; Lu, X.; Zhu, Y.; Xia, Y. Pd-Pt bimetallic nanodendrites with high activity for oxygen reduction. Science 2009, 324, 1302–1305.
Habas, S. E.; Lee, H.; Radmilovic, V.; Somorjai, G. A.; Yang, P. Shaping binary metal nanocrystals through epitaxial seeded growth. Nat. Mater. 2007, 6, 692–697.
LaMer, V. K.; Dinegar, R. H. Theory, production and mechanism of formation of monodispersed hydrosols. J. Am. Chem. Soc. 1950, 72, 4847–4854.
Peng, X.; Wickham, J.; Alivisatos, A. P. Kinetics of II-VI and III-V colloidal semiconductor nanocrystal growth: “Focusing” of size distributions. J. Am. Chem. Soc. 1998, 120, 5343–5344.
Park, J.; Joo, J.; Kwon, S. G.; Jang, Y.; Hyeon, T. Synthesis of monodisperse spherical nanocrystals. Angew. Chem. Int. Ed. 2007, 46, 4630–4660.
Anwar, J.; Boateng, P. K. Computer simulation of crystallization from solution. J. Am. Chem. Soc. 1998, 120, 9600–9604.
Niederberger, M.; Colfen, H. Oriented attachment and mesocrystals: Non-classical crystallization mechanisms based on nanoparticle assembly. Phys. Chem. Chem. Phys. 2006, 8, 3271–3287.
Watzky, M. A.; Finney, E. E.; Finke, R. G. Transition-metal nanocluster size vs. formation time and the catalytically effective nucleus number: A mechanism-based treatment. J. Am. Chem. Soc. 2008, 130, 11959–11969.
Lim, B.; Wang, J.; Camargo, P. H. C.; Cobley, C. M.; Kim, M. J.; Xia, Y. Twin-induced growth of palladium-platinum alloy nanocrystals. Angew. Chem. Int. Ed. 2009, 48, 6304–6308.
Bisson, L.; Boissiere, C.; Nicole, L.; Grosso, D.; Jolivet, J. P.; Thomazeau, C.; Uzio, D.; Berhault, G.; Sanchez, C. Formation of palladium nanostructures in a seed-mediated synthesis through an oriented-attachment-directed aggregation. Chem. Mater. 2009, 21, 2668–2678.
Banfield, J. F.; Welch, S. A.; Zhang, H.; Ebert, T. T.; Penn, R. L. Aggregation-based crystal growth and microstructure development in natural iron oxyhydroxide biomineralization products. Science 2000, 289, 751–754.
Pacholski, C.; Kornowski, A.; Weller, H. Self-assembly of ZnO: From nanodots to nanorods. Angew. Chem. Int. Ed. 2002, 41, 1188–1191.
Tang, Z.; Kotov, N. A.; Giersig, M. Spontaneous organization of single CdTe nanoparticles into luminescent nanowires. Science 2002, 297, 237–240.
Zhang, Z.; Tang, Z.; Kotov, N. A.; Glotzer, S. C. Simulations and analysis of self-assembly of CdTe nanoparticles into wires and sheets. Nano Lett. 2007, 7, 1670–1675.
Yu, J. H.; Joo, J.; Park, H. M.; Baik, S. -I.; Kim, Y. W.; Kim, S. C.; Hyeon, T. Synthesis of quantum-sized cubic ZnS nanorods by the oriented attachment mechanism. J. Am. Chem. Soc. 2005, 127, 5662–5670.
Halder, A.; Ravishankar, N. Ultrafine single-crystalline gold nanowire arrays by oriented attachment. Adv. Mater. 2007, 19, 1854–1858.
Zheng, H.; Smith, R. K.; Jun, Y. -W.; Kisielowski, C.; Dahmen, U.; Alivisatos, A. P. Observation of single colloidal platinum nanocrystal growth trajectories. Science 2009, 324, 1309–1312.
Lim, B.; Jiang, M.; Tao, J.; Camargo, P. H. C.; Zhu, Y.; Xia, Y. Shape-controlled synthesis of Pd nanocrystals in aqueous solutions. Adv. Funct. Mater. 2009, 19, 189–200.
Xiong, Y.; Cai, H.; Wiley, B. J.; Wang, J.; Kim, M. J.; Xia, Y. Synthesis and mechanistic study of palladium nanobars and nanorods. J. Am. Chem. Soc. 2007, 129, 3665–3675.
Niu, W.; Li, Z. -Y.; Shi, L.; Liu, X.; Li, H.; Han, S.; Chen, J.; Xu, G. Seed-mediated growth of nearly monodisperse palladium nanocubes with controllable sizes. Cryst. Growth Des. 2008, 8, 4440–4444.
Zhang, Z.; Lagally, M. G. Atomistic processes in the early stages of thin-film growth. Science 1997, 276, 377–383.
Author information
Authors and Affiliations
Corresponding authors
Additional information
This article is published with open access at Springerlink.com
These two authors contributed equally to this work.
Electronic supplementary material
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Lim, B., Kobayashi, H., Camargo, P.H.C. et al. New insights into the growth mechanism and surface structure of palladium nanocrystals. Nano Res. 3, 180–188 (2010). https://doi.org/10.1007/s12274-010-1021-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-010-1021-5