Abstract
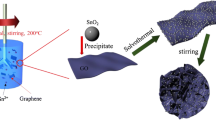
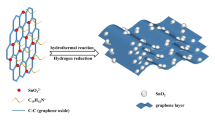
High-rate anode materials for lithium-ion batteries are desirable for applications that require high power density. We demonstrate the advantageous rate capability of few-layered graphene nanosheets, with widths of 100–200 nm, over micro-scale graphene nanosheets. Possible reasons for the better performance of the former include their smaller size and better conductivity than the latter. Combination of SnO2 nanoparticles with graphene was used to further improve the gravimetric capacities of the electrode at high charge-discharge rates. Furthermore, the volumetric capacity of the composites was substantially enhanced compared to pristine graphene due to the higher density of the composites.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Whittingham, M. S. Lithium batteries and cathode materials. Chem. Rev. 2004, 104, 4271–4301.
Kang, K. S.; Meng, Y. S.; Breger, J.; Grey, C. P.; Ceder, G. Electrodes with high power and high capacity for rechargeable lithium batteries. Science 2006, 311, 977–980.
Lee, Y. J.; Yi, H.; Kim, W.; Kang, K.; Yun, D. S.; Strano, M. S.; Ceder, G.; Belcher, A. M. Fabricating genetically engineered high-power lithium-ion batteries using multiple virus genes. Science 2009, 324, 1051–1055.
Levi, M. D.; Aurbach, D. Diffusion coefficients of lithium ions during intercalation into graphite derived from the simultaneous measurements and modeling of electrochemical impedance and potentiostatic intermittent titration characteristics of thin graphite electrodes. J. Phys. Chem. B 1997, 101, 4641–4647.
Bueno, P. R.; Leite, E. R. Nanostructured Li ion insertion electrodes. 1. Discussion on fast transport and short path for ion diffusion. J. Phys. Chem. B 2003, 107, 8868–8877.
Li, N. C.; Martin, C. R.; Scrosati, B. Nanomaterial-based Li-ion battery electrodes. J. Power Sources 2001, 97-98, 240–243.
Sides, C. R.; Martin, C. R. Nanostructured electrodes and the low-temperature performance of Li-ion batteries. Adv. Mater. 2005, 17, 125–128.
Patrissi, C. J.; Martin, C. R. Improving the volumetric energy densities of nanostructured V2O5 electrodes prepared using the template method. J. Electrochem. Soc. 2001, 148, A1247–A1253.
Fu, L. J.; Zhang, T.; Cao, Q.; Zhang, H. P.; Wu, Y. P. Preparation and characterization of three-dimensionally ordered mesoporous titania microparticles as anode material for lithium ion battery. Electrochem. Commun. 2007, 9, 2140–2144.
Hu, Y. S.; Kienle, L.; Guo, Y. G.; Maier, J. High lithium electroactivity of nanometer-sized rutile TiO2. Adv. Mater. 2006, 18, 1421–1426.
Armstrong, A. R.; Armstrong, G.; Canales, J.; Garcia, R.; Bruce, P. G. Lithium-ion intercalation into TiO2-B nanowires. Adv. Mater. 2005, 17, 862–865.
Zaghib, K.; Brochu, F.; Guerfi, A.; Kinoshita, K. Effect of particle size on lithium intercalation rates in natural graphite. J. Power Sources 2001, 103, 140–146.
Hu, Y. S.; Adelhelm, P.; Smarsly, B. M.; Hore, S.; Antonietti, M.; Maier, J. Synthesis of hierarchically porous carbon monoliths with highly ordered microstructure and their application in rechargeable lithium batteries with high-rate capability. Adv. Funct. Mater. 2007, 17, 1873–1878.
Li, N. C.; Mitchell, D. T.; Lee, K. P.; Martin, C. R. A nano-structured honeycomb carbon anode. J. Electrochem. Soc. 2003, 150, A979–A984.
Lee, K. T.; Lytle, J. C.; Ergang, N. S.; Oh, S. M.; Stein, A. Synthesis and rate performance of monolithic macroporous carbon electrodes for lithium-ion secondary batteries. Adv. Funct. Mater. 2005, 15, 547–556.
Zhang, F.; Wang, K. X.; Li, G. D.; Chen, J. S. Hierarchical porous carbon derived from rice straw for lithium ion batteries with high-rate performance. Electrochem. Commun. 2009, 11, 130–133.
Yoo, E.; Kim, J.; Hosono, E.; Zhou, H.; Kudo, T.; Honma, I. Large reversible Li storage of graphene nanosheet families for use in rechargeable lithium ion batteries. Nano Lett. 2008, 8, 2277–2282.
Tatsumi, K.; Iwashita, N.; Sakaebe, H.; Shioyama, H.; Higuchi, S.; Mabuchi, A.; Fujimoto, H. The influence of the graphitic structure on the electrochemical characteristics for the anode of secondary lithium batteries. J. Electrochem. Soc. 1995, 142, 716–720.
Paek, S. M.; Yoo, E.; Honma, I. Enhanced cyclic performance and lithium storage capacity of SnO2/graphene nanoporous electrodes with three-dimensionally delaminated flexible structure. Nano Lett. 2009, 9, 72–75.
Wang, D. H.; Choi, D. W.; Li, J.; Yang, Z. G.; Nie, Z. M.; Kou, R.; Hu, D. H.; Wang, C. M.; Saraf, L. V.; Zhang, J. G.; Aksay, I. A.; Liu, J. Self-assembled TiO2-graphene hybrid nanostructures for enhanced Li-ion insertion. ACS Nano 2009, 3, 907–914.
Li, N.; Wang, Z. Y.; Zhao, K. K.; Shi, Z. J.; Gu, Z. N.; Xu, S. K. Large scale synthesis of N-doped multi-layered graphene sheets by simple arc-discharge method. Carbon 2010, 48, 255–259.
Stankovich, S.; Dikin, D. A.; Piner, R. D.; Kohlhaas, K. A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S. T.; Ruoff, R. S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565.
Habazaki, H.; Kiriu, M.; Konno, H. High rate capability of carbon nanofilaments with platelet structure as anode materials for lithium ion batteries. Electrochem. Commun. 2006, 8, 1275–1279.
Wang, G. X.; Ahn, J. H.; Lindsay, M. J.; Sun, L.; Bradhurst, D. H.; Dou, S. X.; Liu, H. K. Graphite-tin composites as anode materials for lithium-ion batteries. J. Power Sources 2001, 97-98, 211–215.
Wen, Z. H.; Wang, Q.; Zhang, Q.; Li, J. H. In situ growth of mesoporous SnO2 on multiwalled carbon nanotubes: A novel composite with porous-tube structure as anode for lithium batteries. Adv. Funct. Mater. 2007, 17, 2772–2778.
Zhang, W. M.; Hu, J. S.; Guo, Y. G.; Zheng, S. F.; Zhong, L. S.; Song, W. G.; Wan, L. J. Tin-nanoparticles encapsulated in elastic hollow carbon spheres for high-performance anode material in lithium-ion batteries. Adv. Mater. 2008, 20, 1160–1165.
Lou, X. W.; Li, C. M.; Archer, L. A. Designed synthesis of coaxial SnO2@carbon hollow nanospheres for highly reversible lithium storage. Adv. Mater. 2009, 21, 2536–2539.
Courtney, I. A.; Dahn, J. R. Electrochemical and in situ X-ray diffraction studies of the reaction of lithium with tin oxide composites. J. Electrochem. Soc. 1997, 144, 2045–2052.
Sivashanmugam, A.; Kumar, T. P.; Renganathan, N. G.; Gopukumar, S.; Wohlfahrt-Mehrens, M.; Garche, J. Electrochemical behavior of Sn/SnO2 mixtures for use as anode in lithium rechargeable batteries. J. Power Sources 2005, 144, 197–203.
Zhao, Y. M.; Zhou, Q.; Liu, L.; Xu, J.; Yan, M. M.; Jiang, Z. Y. A novel and facile route of ink-jet printing to thin film SnO2 anode for rechargeable lithium ion batteries. Electrochim. Acta 2006, 51, 2639–2645.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published with open access at Springerlink.com
Electronic supplementary material
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Wang, Z., Zhang, H., Li, N. et al. Laterally confined graphene nanosheets and graphene/SnO2 composites as high-rate anode materials for lithium-ion batteries. Nano Res. 3, 748–756 (2010). https://doi.org/10.1007/s12274-010-0041-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-010-0041-5