Abstract
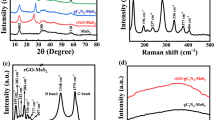
A graphene/TiO2 nanocrystals hybrid has been successfully prepared by directly growing TiO2 nanocrystals on graphene oxide (GO) sheets. The direct growth of the nanocrystals on GO sheets was achieved by a two-step method, in which TiO2 was first coated on GO sheets by hydrolysis and crystallized into anatase nanocrystals by hydrothermal treatment in the second step. Slow hydrolysis induced by the use of EtOH/H2O mixed solvent and addition of H2SO4 facilitates the selective growth of TiO2 on GO and suppresses growth of free TiO2 in solution. The method offers easy access to the GO/TiO2 nanocrystals hybrid with a uniform coating and strong interactions between TiO2 and the underlying GO sheets. The strong coupling gives advanced hybrid materials with various applications including photocatalysis. The prepared graphene/TiO2 nanocrystals hybrid has superior photocatalytic activity to other TiO2 materials in the degradation of rhodamine B, showing an impressive three-fold photocatalytic enhancement over P25. It is expected that the hybrid material could also be promising for various other applications including lithium ion batteries, where strong electrical coupling to TiO2 nanoparticles is essential.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Wang, H. L.; Robinson, J. T.; Diankov, G.; Dai, H. J. Nanocrystal growth on graphene with various degrees of oxidation. J. Am. Chem. Soc. 2010, 132, 3270–3271.
Si, Y. C.; Samulski, E. T. Exfoliated graphene separated by platinum nanoparticles. Chem. Mater. 2008, 20, 6792–6797.
Lee, D. H.; Kim, J. E.; Han, T. H.; Hwang, J. W.; Jeon, S.; Choi, S. Y.; Hong, S. H.; Lee, W. J.; Ruoff, R. S.; Kim, S. O. Versatile carbon hybrid films composed of vertical carbon nanotubes grown on mechanically compliant graphene films. Adv. Mater. 2010, 22, 1247–1252.
Wang, H. L.; Sanchez Casalongue, H.; Liang, Y. Y.; Dai, H. J. Ni(OH)2 nanoplates grown on graphene as advanced electrochemical pseudocapacitor materials. J. Am. Chem. Soc. 2010, 132, 7472–7477.
Yoo, E. J.; Okata, T.; Akita, T.; Kohyama, M.; Nakamura, J.; Honma, I. Enhanced electrocatalytic activity of Pt sub-nanoclusters on graphene nanosheet surface. Nano Lett. 2009, 9, 2255–2259.
Murugan, A. V.; Muraliganth, T.; Manthiram, A. Rapid, facile microwave-solvothermal synthesis of graphene nanosheets and their polyaniline nanocomposites for energy storage. Chem. Mater. 2009, 21, 5004–5006.
Williams, G.; Seger, B.; Kamat, P. V. TiO2-graphene nanocomposites. UV-assisted photocatalytic reduction of graphene oxide. ACS Nano 2008, 2, 1487–1491.
Wang, D. H.; Kou, R.; Choi, D. W.; Yang, Z. G.; Nie, Z. M.; Li, J.; Saraf, L. V.; Hu, D. H.; Zhang, J. G.; Graff, G. L.; Liu, J.; Pope, M. A.; Aksay, I. A. Ternary self-assembly of ordered metal oxide-graphene nanocomposites for electro-chemical energy storage. ACS Nano, 2010, 4, 1587–1595.
Wang, X. R.; Tabakman, S. M.; Dai, H. J. Atomic layer deposition of metal oxides on pristine and functionalized graphene. J. Am. Chem. Soc. 2008, 130, 8152–8153.
Lu, G. H.; Mao, S.; Park, S.; Ruoff, R. S.; Chen, J. H. Facile, noncovalent decoration of graphene oxide sheets with nano-crystals. Nano Res. 2009, 2, 192–200.
Chen, X. B.; Mao, S. S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959.
Wang, D. H.; Choi, D. W.; Li, J.; Yang, Z. G.; Nie, Z. M.; Kou, R.; Hu, D. H.; Wang, C. M.; Saraf, L. V.; Zhang, J. G.; Aksay, I. A.; Liu, J. Self-assembled TiO2-graphene hybrid nanostructures for enhanced Li-ion insertion. ACS Nano, 2009, 3, 907–914.
Zhang, H.; Lv, X. J.; Li, Y. M.; Wang, Y.; Li, J. H. P25-graphene composite as a high performance photocatalyst. ACS Nano 2010, 4, 380–386.
Yang, N. L.; Zhai, J.; Wang, D.; Chen, Y. S.; Jiang, L. Two-dimensional graphene bridges enhanced photoinduced charge transport in dye-sensitized solar cells. ACS Nano, 2010, 4, 887–894.
Hummer, W. S.; Offeman, R. E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339–1339.
Wang, H. L.; Wang, X. R.; Li, X. L.; Dai, H. J. Chemical self-assembly of graphene sheets. Nano Res. 2009, 2, 336–342.
Sun, X. M.; Liu, Z.; Welsher, K.; Robinson, J. T.; Goodwin, A.; Zaric, S.; Dai, H. J. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008, 1, 203–212.
Wang, H. L.; Robinson, J. T.; Li, X. L.; Dai, H. J. Solvothermal reduction of chemically exfoliated graphene sheets. J. Am. Chem. Soc. 2009, 131, 9910–9911.
Ravelli, D.; Dondi, D.; Fagnoni, M.; Albini, A. Photocatalysis. A multi-faceted concept for green chemistry. Chem. Soc. Rev. 2009, 38, 1999–2011.
Woan, K.; Pyrgiotakis, G.; Sigmund, W. Photocatalytic carbon-nanotube-TiO2 composites. Adv. Mater. 2009, 21, 2233–2239.
Wang, X. H.; Li, J. G.; Kamiyama, H.; Moriyoshi, Y.; Ishigaki, T. Wavelength-sensitive photocatalytic degradation of methyl orange in aqueous suspension over iron(III)-doped TiO2 nanopowders under UV and visible light irradiation. J. Phys. Chem. B, 2006, 110, 6804–6809.
Wang, W. D.; Serp, P.; Kalck, P.; Faria, J. L. Photocatalytic degradation of phenol on MWNT and titania composite catalysts prepared by a modified sol-gel method. Appl. Catal. B 2005, 56, 305–312.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published with open access at Springerlink.com
These authors contributed equally.
Electronic supplementary material
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Liang, Y., Wang, H., Sanchez Casalongue, H. et al. TiO2 nanocrystals grown on graphene as advanced photocatalytic hybrid materials. Nano Res. 3, 701–705 (2010). https://doi.org/10.1007/s12274-010-0033-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-010-0033-5