Abstract
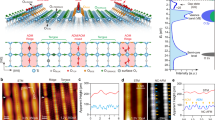
Scanning tunneling microscopy/spectroscopy (STM/STS) at 4.8 K has been used to examine the growth of a double-decker bis(phthalocyaninato)yttrium (YPc2) molecule on a reconstructed Au(111) substrate. Local differential conductance spectra (dI/dV) of a single YPc2 molecule allow the characteristics of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) to be identified. Furthermore, lateral distributions of the local density of states (LDOS) have also been obtained by dI/dV mapping and confirmed by first principles simulations. These electronic feature mappings and theoretical calculations provide a basis for understanding the unique STM morphology of YPc2, which is usually imaged as an eight-lobed structure. In addition, we demonstrate that bias-dependent STM morphologies and simultaneous dI/dV maps can provide a way of understanding the stability of two-dimensional YPc2 films.

Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Joachim, C.; Gimzewski, J. K.; Aviram, A. Electronics using hybrid-molecular and mono-molecular devices. Nature 2000, 408, 541–548.
Cracium, M. F.; Rogge, S.; Morpurgo, A. F. Correlation between molecular orbitals and doping dependence of the electrical conductivity in electron-doped metal-phthalocyanine compounds. J. Am. Chem. Soc. 2005, 127, 12210–12211.
Papageorgiou, N.; Salomon, E.; Angot, T.; Layet, J. M.; Giovanelli, L.; Lay, G. L. Physics of ultra-thin phthalocyanine films on semiconductors. Prog. Surf. Sci. 2004, 77, 139–170.
Lippel, P. H.; Wilson, R. J.; Miller, M. D.; Woll, Ch.; Chiang, S. High-resolution imaging of copper-phthalocyanine by scanning-tunneling microscopy. Phys. Rev. Lett. 1989, 62, 171–174.
Hipps, K. W.; Lu, X.; Wang, X. D.; Mazur, U. Metal d-orbital occupation-dependent images in the scanning tunneling microscopy of metal phthalocyanines. J. Phys. Chem. 1996, 100, 11207–11210.
Lu, X.; Hipps, K. W.; Wang, X. D.; Mazur, U. Scanning tunneling microscopy of metal phthalocyanines: d7 and d9 cases. J. Am. Chem. Soc. 1996, 118, 7197–7202.
Chizhov, I.; Scoles, G.; Kahn, A. The influence of steps on the orientation of copper phthalocyanine monolayers on Au(111). Langmuir 2000, 16, 4358–4361.
Takada, M.; Tada, H. Scanning tunneling microscopy and spectroscopy of phthalocyanine molecules on metal surfaces. Jpn. J. Appl. Phys. 2005, 44, 5332–5335.
Chen, L.; Hu, Z. P.; Zhao, A. D.; Wang, B.; Luo, Y.; Yang, J. L.; Hou, J. G. Mechanism for negative differential resistance inmolecular electronic devices: Local orbital symmetry matching. Phys. Rev. Lett. 2007, 99, 146803.
Zhao, A. D.; Li, Q. X.; Chen, L.; Xiang, H. J.; Wang, W. H.; Pan, S. A.; Wang, B.; Xiao, X. D.; Yang, J. L.; Hou, J. G.; Zhu, Q. S. Controlling the Kondo effect of an adsorbed magnetic ion through its chemical bonding. Science 2005, 309, 1542–1544.
Jiang, P.; Ma, X. C.; Ning, Y. X.; Song, C. L.; Chen, X.; Jia, J. F., Xue, Q. K. Quantum size effect directed selective self-assembling of cobalt phthalocyanine on Pb(111) thin films. J. Am. Chem. Soc. 2008, 130, 7790–7791.
Cheng, Z. H.; Gao, L.; Deng, Z. T.; Jiang, N.; Liu, Q.; Shi, D. X.; Du, S. X.; Guo, H. M.; Gao, H. J. Adsorption behavior of iron phthalocyanine on Au(111) surface at submonolayer coverage. J. Phys. Chem. C 2007, 111, 9240–9244.
Strohmaier, R.; Ludwig, C.; Petersen, J.; Gompf, B.; Eisenmenger, W. Scanning tunneling microscope investigations of lead-phthalocyanine on MoS2. J. Vac. Sci. Technol. B 1996, 14, 1079–1082.
Gopakumar, T. G.; Lackinger, M.; Hackert, M.; Muller, F.; Hietschold, M. Adsorption of palladium phthalocyanine on graphite: STM and LEED study. J. Phys. Chem. B 2004, 108, 7839–7843.
Fu, Y. S.; Ji, S. H.; Chen, X.; Ma, X. C.; Wu, R.; Wang, C. C.; Duan, W. H.; Qiu, X. H.; Sun, B.; Zhang, P.; Jia, J. F.; Xue, Q. K. Manipulating the Kondo resonance through 1uantum size effects. Phys. Rev. Lett. 2007, 99, 256601.
Gao, L.; Ji, W.; Hu, Y. B.; Cheng, Z. H.; Deng, Z. T.; Liu, Q.; Jiang, N.; Lin, X.; Guo, W.; Du, S. X.; Hofer, W. A.; Xie, X. C.; Gao, H. J. Site-specific Kondo effect at ambient temperatures in iron-based molecules. Phys. Rev. Lett. 2007, 99, 106402.
Nazin, G. V.; Qiu, X. H.; Ho, W. Visualization and spectroscopy of a metal.molecule.metal bridge. Science 2003, 302, 77–81.
Qiu, X. H.; Nazin, G. V.; Ho, W. Vibronic states in single molecule electron transport. Phys. Rev. Lett. 2004, 92, 206102.
Nilius, N.; Simic-Milosevic, V. Adsorption of single magnesium phthalocyanine molecules on V2O3 thin films. J. Phys. Chem. C 2008, 112, 10027–10031.
Yang, Z. Y.; Gan, L. H.; Lei, S. B.; Wan, L. J.; Wang, C.; Jiang, J. Z. Self-assembly of PcOC8 and its sandwich lanthanide complex Pr(PcOC8)2 with oligo(phenylene-ethynylene) molecules. J. Phys. Chem. B 2005, 109, 19859–19865.
Takami, T.; Arnold, D. P.; Fuchs, A. V.; Will, G. D.; Goh, R.; Waclawik, E. R.; Bell, J. M.; Weiss, P. S.; Sugiura, K.; Liu, W.; Jiang, J. Two-dimensional crystal growth and stacking of bis(phthalocyaninato) rare earth sandwich complexes at the 1-phenyloctane/graphite interface. J. Phys. Chem B. 2006, 110, 1661–1664.
Lei, S. B.; Deng, K.; Yang, Y. L.; Zeng, Q. D.; Wang, C.; Jiang, J. Z. Electric driven molecular switching of asymmetric tris(phthalocyaninato) lutetium triple-decker complex at theliquid/solid interface. Nano. Lett. 2008, 8, 1836–1843.
Vitali, L.; Fabris, S.; Conte, A. M.; Brink, S.; Ruben, M.; Baroni, S.; Kern, K. Electronic structure of surface-supported bis(phthalocyaninato) terbium(III) single molecular magnets. Nano. Lett. 2008, 8, 3364–3368.
Zhang, Y, F.; Isshiki, H.; Katoh, K.; Yoshida, Y.; Yamashita, M.; Miyasaka, H.; Breedlove, B. K.; Kajiwara, T.; Takaishi, S.; Komeda, T. Low-temperature scanning tunneling microscopy investigation of bis(phthalocyaninato)yttrium growth on Au(111): From individual molecules to two-dimensional domains. J. Phys. Chem. C 2009, 113, 9826–9830.
Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775.
Perdew, J. P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868.
Kresse, G.; Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50.
Katoh, K.; Yoshida, Y.; Yamashita, M.; Miyasaka, H.; Breedlove, B. K.; Kajiwara, T.; Takaishi, S.; Ishikawa, N.; Isshiki, H.; Zhang, Y. F.; Komeda, T.; Yamagishi, M.; Takeya, J. Direct observation of lanthanide(III)-phthalocyanine molecules on Au(111) by using scanning tunneling microscopy and scanning tunneling spectroscopy and thin-film field-effect transistor properties of Tb(III)- and Dy(III)-phthalocyanine molecules. J. Am. Chem. Soc. 2009, 131, 9967–9976.
Author information
Authors and Affiliations
Corresponding authors
Additional information
This article is published with open access at Springerlink.com
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Zhang, Y., Guan, P., Isshiki, H. et al. Bis(phthalocyaninato)yttrium grown on Au(111): Electronic structure of a single molecule and the stability of two-dimensional films investigated by scanning tunneling microscopy/spectroscopy at 4.8 K. Nano Res. 3, 604–611 (2010). https://doi.org/10.1007/s12274-010-0021-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-010-0021-9