Abstract
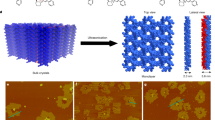
Arrays of low-dimensional molecular crystals of square columns (1-D) and nanolamellae (2-D) of Zn[TCNQ]2(H2O)2 with large areas (up to 10 20 cm2) have been synthesized by controlled addition of water to Zn and TCNQ. Based on the ability to accurately control the reaction, a new moisture and water indicator has been developed. The simple method, the large areas of material prepared, the fine size tuning, and the typical semiconductor behavior of the resulting low-dimensional molecular materials promise applications in molecular electronics as well as nanoelectronics. The system is an effective indicator for the detection of traces of water and moisture.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Acker, D. S.; Harder, R. J.; Hertler, W. R.; Mahler, W.; Melby, L. R.; Benson, R. E.; Mochel, W. E. 7,7,8,8-Tetracyanoquinodimethane and its electrically conducting anion-radical derivatives. J. Am. Chem. Soc. 1960, 82, 6408–6409.
Wheland, R. C.; Gillson, J. L. Synthesis of electrically conductive organic solids. J. Am. Chem. Soc. 1976, 98, 3916–3925.
Kathirgamanathan, P.; Rosseinsky, D. R. Electrocrystallized metal-tetracyanoquinodimethane salts with high electrical-conductivity. J. Chem. Soc., Chem. Commun. 1980, 17, 839–840.
Bolinger, C. M.; Darkwa, J.; Gammie, G.; Gammon, S. D.; Lyding, J. W.; Rauchfuss, T. B.; Wilson, S. R. Synthesis, structure, and electrical properties of [(MeCp)5V5S6][(TCNQ)2]. Organomet. 1986, 5, 2386–2388.
Kulys, J.; Drungiliene, A. Electrocatalytic oxidation of ascorbic acid at chemically modified electrodes. Electroanal. 1991, 3, 209–214.
Murthy, A. S. N.; Anita, G. R. L. NADH sensor with electrochemically modified TCNQ electrode. Anal. Chim. Acta 1994, 289, 43–46.
Wooster, T. J.; Bond, A. M.; Honeychurch, M. J. An analogy of an ion-selective electrode sensor based on the voltammetry of microcrystals of tetracyanoquinodimethane or tetrathiafulvalene adhered to an electrode surface. Anal. Chem. 2003, 75, 586–592.
Wooster, T. J.; Bond, A. M. Ion selectivity obtained under voltammetric conditions when a TCNQ chemically modified electrode is presented with aqueous solutions containing tetraalkylammonium cations. Analyst 2003, 128, 1386–1390.
Okamoto, T.; Kozaki, M.; Doe, M.; Uchida, M.; Wang, G.; Okada, K. 1,4-Benzoxazino[2,3-b]phenoxazine and its sulfur analogues: Synthesis, properties, and application to organic light-emitting diodes. Chem. Mater. 2005, 17, 5504–5511.
Mueller, R.; Jonge, S. D.; Myny, K.; Wouters, D. J.; Genoe, J.; Heremans, P. Organic CuTCNQ non-volatile memories for integration in the CMOS backend-of-line: Preparation from gas/solid reaction and downscaling to an area of 0.25 µm2. Solid State Electron. 2006, 50, 601–605.
Heintz, R. A.; Zhao, H.; Ouyang, X.; Grandinetti, G.; Cowen, J.; Dunbar, K. R. New insight into the nature of Cu(TCNQ): Solution routes to two distinct polymorphs and their relationship to crystalline films that display bistable switching behavior. Inorg. Chem. 1999, 38, 144–156.
Neufeld, A. K.; Madsen, I.; Bond, A. M.; Hogan, C. F. Phase, morphology, and particle size changes associated with the solid solid electrochemical interconversion of TCNQ and semiconducting CuTCNQ (TCNQ = tetracyanoquinodimethane). Chem. Mater. 2003, 15, 3573–2585.
Neufeld, A. K.; O’Mullane, A. P.; Bond, A. M. Control of localized nanorod formation and patterns of semiconducting CuTCNQ phase I crystals by scanning electrochemical microscopy. J. Am. Chem. Soc. 2005, 127, 13846–13853.
O’Mullane, A. P.; Neufeld, A. K.; Bond, A. M. Distinction of the two phases of CuTCNQ by scanning electrochemical microscopy. Anal. Chem. 2005, 77, 5447–5452.
O’Mullane, A. P.; Neufeld, A. K.; Harris, A. R.; Bond, A. M. Electrocrystallization of phase I, CuTCNQ (TCNQ = 7,7,8,8-tetracyanoquinodimethane), on indium tin oxide and boron-doped diamond electrodes. Langmuir 2006, 22, 10499–10505.
Siedle, A. R.; Candela, G. A.; Finnegan, T. F. Transitionmetal derivatives of the teracyanoquinodimethane ion, TCNQ2−. Inorg. Chim. Acta 1979, 35, 125–130.
Nafady, A.; O’Mullane, A. P.; Bond, A. M.; Neufeld, A. K. Morphology changes and mechanistic aspects of the electrochemically-induced reversible solid solid transformation of microcrystalline TCNQ into Co[TCNQ]2-based materials (TCNQ = 7,7,8,8-tetracyanoquino dimethane). Chem. Mater. 2006, 18, 4375–4384.
Clerac, R.; O’Kane, S.; Cowen, J.; Ouyang, X.; Heintz, R.; Zhao, H.; Bazile, M. J.; Dunbar, Jr. K. R. Glassy magnets composed of metals coordinated to 7,7,8,8-tetracyanoq uinodimethane: M(TCNQ)2 (M = Mn, Fe, Co, Ni). Chem. Mater. 2003, 15, 1840–1850.
Nafady, A.; Bond, A. M.; Bilyk, M. A.; Harris, A. R.; Bhatt, A. I.; O’Mullane, A. P.; Marco, R. D. Tuning the electrocrystallization parameters of semiconducting Co[TCNQ]2-based materials to yield either single nanowires or crystalline thin films. J. Am. Chem. Soc. 2007, 129, 2369–2382.
Goh, S. H.; Lee, S. Y.; Zhou, X.; Tan, K. L. X-ray photoelectron spectroscopic studies of interactions between poly(4-vinylpyridine) and poly(styrenesulfonate) salts. Macromolecules 1998, 31, 4260–4264.
Potember, R. S.; Poehler, T. O.; Cowan, D. O.; Carter, F. L.; Brant, P. I. In Molecular Electronic Devices; Carter, F. L., Eds.; Marcel Dekker: New York, 1982.
Ikemoto, I.; Thomas, J. M.; Kuroda, H. X-ray photoelectron spectra of copper-tetracyanoquinodimethane complexes. Bull. Chem. Soc. Jpn. 1973, 46, 2237–2238.
Khatkale, K. S.; Devlin, J. P. The vibrational and electronic spectra of the mono-, di-, and trianon salts of TCNQ. J. Chem. Phys. 1979, 70, 1851–1859.
Zhao, H.; Heintz, R. A.; Ouyang, X.; Dunbar, K. R.; Campana, C. F.; Rogers, R. D. Spectroscopic, thermal, and magnetic properties of metal/TCNQ network polymers with extensive supramolecular interactions between layers. Chem. Mater. 1999, 11, 736–746.
Melby, L. R.; Harder, R. J.; Hertler, W. R.; Mahler, W.; Benson R. E. Substituted quinodimethans, II. Anionradical derivatives and complexes of 7,7,8,8-tetracyano quinodimethan. J. Am. Chem. Soc. 1962, 84, 3374–3387.
Jeanmaire, D. L.; van Duyne, R. P. Resonance Raman spectroelectrochemistry. 2. Scattering spectroscopy accompanying excitation of the lowest 2B1u excited state of the tetracyanoquinodimethane anion radical. J. Am. Chem. Soc. 1976, 98, 4029–4033.
Gong, J. P.; Osada, Y. Preparation of polymeric metaltetracyanoquinodimethane film and its bistable switching. Appl. Phys. Lett. 1992, 61, 2787–2789.
Liu, S.; Liu, Y.; Wu, P.; Zhu, D. Multifaceted study of CuTCNQ thin-film materials. Fabrication, morphology, and spectral and electrical switching properties. Chem. Mater. 1996, 8, 2779–2787.
Liu, Y.; Ji, Z.; Tang, Q.; Jiang, L.; Li, H.; He, M.; Hu, W.; Zhang, D.; Jiang, L.; Wang, X.; Wang, C.; Liu, Y.; Zhu, D. Particle-size control and patterning of a charge-transfer complex for nanoelectronics. Adv. Mater. 2005, 17, 2953–2958.
Tang, Q.; Li, H.; He, M.; Hu, W.; Liu, C.; Chen, K.; Wang, C.; Liu, Y.; Zhu, D. Low threshold voltage transistors based on individual single-crystalline submicrometersized ribbons of copper phthalocyanine. Adv. Mater. 2006, 18, 65–68.
Lampert, M. A.; Mark, P. Current Injection in Solids; Academic Press: New York and London, 1970.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Ji, Z., Dong, H., Liu, M. et al. Water-controlled synthesis of low-dimensional molecular crystals and the fabrication of a new water and moisture indicator. Nano Res. 2, 857–864 (2009). https://doi.org/10.1007/s12274-009-9084-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-009-9084-x