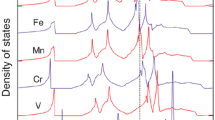
Abstract
We present a brief review of the most important efforts aimed at simulating single-walled carbon nanotube (SWNT) nucleation and growth processes using molecular dynamics (MD) techniques reported in the literature. MD simulations allow the spatio-temporal movement of atoms during nonequilibrium growth to be followed. Thus, it is hoped that a successful MD simulation of the entire SWNT formation process will assist in the design of chirality-specific SWNT synthesis techniques. We give special consideration to the role of the metal catalyst particles assumed in standard theories of SWNT formation, and describe the actual metal behavior observed in the reported MD simulations, including our own recent quantum chemical MD simulations. It is concluded that the use of a quantum potential is essential for a qualitatively correct description of the catalytic behavior of the metal cluster, and that carbide formation does not seem to be a necessary requirement for nucleation and growth of SWNTs according to our most recent quantum chemical MD simulations.

Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605.
Bethune, D. S.; Kiang, C. H.; Devries, M. S.; Gorman, G.; Savoy, R.; Vazquez, J.; Beyers, R. Cobalt-catalyzed growth of carbon nanotubes with single-atomic-layer walls. Nature 1993, 363, 605–607.
Kroto, H. W.; Heath, J. R.; O’Brien, S. C.; Curl, R. F.; Smalley, R. E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163.
Novoselov, K. S.; Geim, A. K.; Morozov, S. V.; Jiang, D.; Zhang, Y.; Dubonos, S. V.; Grivorieva, L. V.; Firsov, A. A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669.
Saito, R.; Dresselhaus, G.; Dresselhaus, M. S. Physical Properties of Carbon Nanotubes; Imperial College Press: London, 1998.
Dresselhaus, M. S.; Dresselhaus, G.; Avouris, P. Carbon Nanotubes: Synthesis, Structure, Properties, and Applications; Springer: New York, 2001.
Baughman, R. A.; Zakhidov, A. A.; de Heer, W. A. Carbon nanotubes—The route toward applications. Science 2002, 297, 787–792.
Palmer, D. J. Where nano is going. Nano Today 2008, 3, 46–47.
Journet, C.; Maser, W. K.; Bernier, P.; Loiseau, A.; Lamy de la Chapelle, M.; Lefrant, S.; Deniard, P.; Lee, R.; Fischer, J. E. Large-scale production of single-walled carbon nanotubes by the electric-arc technique. Nature 1997, 388, 756–758.
Moisala, A.; Nasibulin, A. G.; Kauppinen, E. I. The role of metal nanoparticles in the catalytic production of singlewalled carbon nanotubes—A review. J. Phys. Cond. Matter 2003, 15, S3011–S3035.
Harris, P. J. F. Solid state growth mechanisms for carbon nanotubes. Carbon 2007, 45, 229–239.
Esconjauregui, S.; Whelan, C. M.; Maex, K. The reasons why metals catalyze the nucleation and growth of carbon nanotubes and other carbon nanomorphologies. Carbon 2009, 47, 659–669.
Takagi, D.; Homma, Y.; Hibino, H.; Suzuki, S.; Kobayashi, Y. Single-walled carbon nanotube growth from highly activated metal nanoparticles. Nano Lett. 2006, 6, 2642–2645.
Thess, A.; Lee, R.; Nikolaev, P.; Dai, H. J.; Petit, P.; Robert, J.; Xu, C. H.; Lee, Y. H.; Kim, S. G.; Rinzler, A. G.; Colbert, D. T.; Scuseria, G. E.; Tomanek, D.; Fischer, J. E.; Smalley, R. E. Crystalline ropes of metallic carbon nanotubes. Science 1996, 273, 483–487.
Yudasaka, M.; Yamada, R.; Sensui, N.; Wilkins, T.; Ichihashi, T.; Iijima, S. Mechanism of the effect of NiCo, Ni and Co catalysts on the yield of single-wall carbon nanotubes formed by pulsed Nd:YAG laser ablation. J. Phys. Chem. B 1999, 103, 6224–6229.
Kusunoki, M.; Suzuki, T.; Hirayama, M.; Shibata, N.; Kaneko, K. A formation mechanism of carbon nanotube films on SiC(0001). Appl. Phys. Lett. 2000, 77, 531–533.
Takagi, D.; Hibino, H.; Suzuki, S.; Kobayashi, Y.; Homma, Y. Carbon nanotube growth from semiconductor nanoparticles. Nano Lett. 2007, 7, 2272–2275.
Huang, S.; Cai, Q.; Chen, J.; Qian, Y.; Zhang, L. Metal-catalyst-free growth of single-walled carbon nanotubes on substrates. J. Am. Chem. Soc. 2009, 131, 2094–2095.
Liu, B.; Ren, W.; Gao, L.; Li, S.; Pei, S.; Liu, C.; Jiang, C.; Cheng, H. M., Metal-catalyst-free growth of single-walled carbon nanotubes. J. Am. Chem. Soc. 2009, 131, 2082–2083.
Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58.
Nikolaev, P.; Bronikowski, M. J.; Bradley, R. K.; Rohmund, F.; Colbert, D. T.; Smith, K. A.; Smalley, R. E. Gas-phase catalytic growth of single-walled carbon nanotubes from carbon monoxide. Chem. Phys. Lett. 1999, 313, 91–97.
Cheng, H. M.; Li, F.; Sun, X.; Brown, S. D. M.; Pimenta, M. A.; Marucci, A.; Dresselhaus, G.; Dresselhaus, M. S. Bulk morphology and diameter distribution of single-walled carbon nanotubes synthesized by catalytic decomposition of hydrocarbons. Chem. Phys. Lett. 1998, 289, 602–610.
Cassell, A. M.; Raymakers, J. A.; Kong, J.; Dai, H. Large scale CVD synthesis of single-walled carbon nanotubes. J. Phys. Chem. B 1999, 103, 6484–6492.
Maruyama, S.; Kojima, R.; Miyauchi, Y.; Chiashi, S.; Kohno, M. Low-temperature synthesis of high-purity single-walled carbon nanotubes from alcohol. Chem. Phys. Lett. 2002, 360, 229–234.
Reich, S.; Li, L.; Robertson, J. Structure and formation energy of carbon nanotube caps. Phys. Rev. B 2005, 72, 165423.
Gomez-Gualdron, D. A.; Balbuena, P. B. The role of cap chirality in the mechanism of growth of single-wall carbon nanotubes. Nanotechnology 2008, 19, 485604.
Yoshida, H.; Takeda, S.; Uchiyama, T.; Kohno, H.; Homma, Y. Atomic-scale in situ observation of carbon nanotube growth from solid state iron carbide nanoparticles. Nano Lett. 2008, 8, 2082–2086.
Hofmann, S.; Sharma, R.; Ducati, C.; Du, G.; Mattevi, C.; Cepek, C.; Cantoro, M.; Pisana, S.; Parvez, A.; Cervantes-Sodi, F.; Ferrari, A. C.; Dunin-Borkowski, R.; Lizzit, S.; Petaccia, L.; Goldoni, A.; Robertson, J. In situ observations of catalyst dynamics during surface-bound carbon nanotube nucleation. Nano Lett. 2007, 7, 602–608.
Jiang, K. Super-aligned carbon nanotube arrays: Controlled synthesis, physical properties and applications. IUMRS International Conference in Asia (IUMRS-ICA), Nagoya, Japan, 2008.
Brenner, D. W. Empirical potential for hydrocarbons for use in simulating the chemical vapor-deposition of diamond films. Phys. Rev. B 1990, 42, 9458–9471.
Brenner, D. W. Empirical potential for hydrocarbons for use in simulating the chemical vapor deposition of diamond films. [Erratum to document cited in CA114(6):53045x]. Phys. Rev. B 1992, 46, 1948.
Brenner, D. W.; Shenderova, O. A.; Harrison, J. A.; Stuart, S. J.; Ni, B.; Sinnott, S. B. A second-generation reactive empirical bond order (REBO) potential energy expression for hydrocarbons. J. Phys. Cond. Matter 2002, 14, 783–802.
Car, R.; Parrinello, M. Unified approach for molecular-dynamics and density-functional theory. Phys. Rev. Lett. 1985, 55, 2471–2474.
Porezag, D.; Frauenheim, T.; Kohler, T.; Seifert, G.; Kaschner, R. Construction of tight-binding-like potentials on the basis of density-functional theory—Application to carbon. Phys. Rev. B 1995, 51, 12947–12957.
Elstner, M.; Porezag, D.; Jungnickel, G.; Elsner, J.; Haugk, M.; Frauenheim, T.; Suhai, S.; Seifert, G. Self-consistent-charge density-functional tight-binding method for simulations of complex materials properties. Phys. Rev. B 1998, 58, 7260–7268.
Dai, H.; Rinzler, A. G.; Nikolaev, P.; Thess, A.; Colbert, D. T.; Smalley, R. E. Single-wall nanotubes produced by metal-catalyzed disproportionation of carbon monoxide. Chem. Phys. Lett. 1996, 260, 471–475.
Gavillet, J.; Loiseau, A.; Journet, C.; Willaime, F.; Ducastelle, F.; Charlier, J. -C. Root-growth mechanism for single-wall carbon nanotubes. Phys. Rev. Lett. 2001, 87, 275504.
Tersoff, J. New empirical approach for the structure and energy of covalent systems. Phys. Rev. B 1988, 37, 6991–7000.
Tersoff, J. Modeling solid-state chemistry: Interatomic potentials for multicomponent systems. Phys. Rev. B 1989, 39, 5566–5568.
Marks, N. A.; Cooper, N. C.; McKenzie, D. R.; McCulloch, D. G.; Bath, P.; Russo, S. P. Comparison of density-functional, tight-binding, and empirical methods for the simulation of amorphous carbon. Phys. Rev. B 2002, 65, 075411.
Zheng, G. S.; Irle, S.; Elstner, M.; Morokuma, K. Quantum chemical molecular dynamics model study of fullerene formation from open-ended carbon nanotubes. J. Phys. Chem. A 2004, 108, 3182–3194.
Irle, S.; Zheng, G.; Wang, Z.; Morokuma, K. The C60 formation puzzle “solved“: QM/MD simulations reveal the shrinking hot giant road of the dynamic fullerene self-assembly mechanism. J. Phys. Chem. B 2006, 110, 14531–14545.
Shibuta, Y.; Maruyama, S. Molecular dynamics simulation of generation process of SWNTs. Physica B 2002, 323, 187–189.
Shibuta, Y.; Maruyama, S. Molecular dynamics simulation of formation process of single-walled carbon nanotubes by CCVD method. Chem. Phys. Lett. 2003, 382, 381–386.
Shibuta, Y.; Maruyama, S. A molecular dynamics study of the effect of a substrate on catalytic metal clusters in nucleation process of single-walled carbon nanotubes. Chem. Phys. Lett. 2007, 437, 218–223.
Ding, F.; Bolton, K.; Rosen, A. Nucleation and growth of single-walled carbon nanotubes: A molecular dynamics study. J. Phys. Chem. B 2004, 108, 17369–17377.
Ding, F.; Rosen, A.; Bolton, K. Molecular dynamics study of the catalyst particle size dependence on carbon nanotube growth. J. Chem. Phys. 2004, 121, 2775–2779.
Ding, F.; Bolton, K.; Rosen, A. Iron-carbide cluster thermal dynamics for catalyzed carbon nanotube growth. J. Vac. Sci. Technol. A 2004, 22, 1471–1476.
Ding, F.; Bolton, K.; Rosen, A. Molecular dynamics study of SWNT growth on catalyst particles without temperature gradients. Comput. Mater. Sci. 2006, 35, 243–246.
Ding, F.; Rosen, A.; Curtarolo, S.; Bolton, K. Modeling the melting of supported clusters. Appl. Phys. Lett. 2006, 88, 133110.
Ding, F.; Larsson, P.; Larsson, J. A.; Ahuja, R.; Duan, H.; Rosen, A.; Bolton, K. The importance of strong carbonmetal adhesion for catalytic nucleation of single-walled carbon nanotubes. Nano Lett. 2008, 8, 463–468.
Shibuta, Y.; Maruyama, S. Bond-order potential for transition metal carbide cluster for the growth simulation of a single-walled carbon nanotube. Comput. Mater. Sci. 2007, 39, 842–848.
Raty, J. Y.; Gygi, F.; Galli, G. Growth of carbon nanotubes on metal nanoparticles: A microscopic mechanism from ab initio molecular dynamics simulations. Phys. Rev. Lett. 2005, 95, 096103.
Raty, J. Y.; Galli, G.; Bostedt, C.; van Buuren, T. W.; Terminello, L. J. Quantum confinement and fullerenelike surface reconstructions in nanodiamonds. Phys. Rev. Lett. 2003, 90, 037401.
Thiel, W.; Voityuk, A. A. Extension of the MNDO formalism to d orbitals: Integral approximations and preliminary numerical results. Theor. Chim. Acta 1996, 93, 315.
Stewart, J. J. P. MOPAC2009, Cache Software Group: 2009.
Zheng, G. S.; Witek, H. A.; Bobadova-Parvanova, P.; Irle, S.; Musaev, D. G.; Prabhakar, R.; Morokuma, K. Parameter calibration of transition-metal elements for the spin-polarized self-consistent-charge density-functional tight-binding (DFTB) method: Sc, Ti, Fe, Co, and Ni. J. Chem. Theory Comput. 2007, 3, 1349–1367.
Weinert, M.; Davenport, J. W. Fractional occupations and density-functional energies and forces. Phys. Rev. B 1992, 45, 13709–13712.
Ohta, Y.; Irle, S.; Okamoto, Y.; Morokuma, K. Rapid growth of a single-walled carbon nanotube on an iron cluster: Density-functional tight-binding molecular dynamics simulations. ACS Nano 2008, 2, 1437–1444.
Wang, Y.; Kim, M. J.; Shan, H.; Kittrell, C.; Fan, H.; Ericson, L. M.; Hwang, W.-F.; Arepalli, S.; Hauge, R. H.; Smalley, R. E. Continued growth of single-walled carbon nanotubes. Nano Lett. 2005, 5, 997–1002.
Smalley, R. E.; Li, Y. B.; Moore, V. C.; Price, B. K.; Colorado, R.; Schmidt, H. K.; Hauge, R. H.; Barron, A. R.; Tour, J. M. Single wall carbon nanotube amplification: En route to a type-specific growth mechanism. J. Am. Chem. Soc. 2006, 128, 15824–15829.
Ohta, Y.; Okamoto, Y.; Irle, S.; Morokuma, K. Temperature dependence of iron-catalyzed continued single-walled carbon nanotube growth rates: Density functional tight-binding molecular dynamics simulations. J. Phys. Chem. C 2009, 113, 159–169.
Ohta, Y.; Okamoto, Y.; Irle, S.; Morokuma, K. Density-functional tight-binding molecular dynamics simulations of SWCNT growth by surface carbon diffusion on an iron cluster. Carbon 2009, 47, 1270–1275.
Larsson, P.; Larsson, J. A.; Ahuja, R.; Ding, F.; Yakobson, B. I.; Duan, H. M.; Rosen, A.; Bolton, K. Calculating carbon nanotube-catalyst adhesion strengths. Phys. Rev. B 2007, 75, 115419.
Zhu, W.; Rosen, A.; Bolton, K. Changes in single-walled carbon nanotube chirality during growth and regrowth. J. Chem. Phys. 2008, 128, 124708.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Irle, S., Ohta, Y., Okamoto, Y. et al. Milestones in molecular dynamics simulations of single-walled carbon nanotube formation: A brief critical review. Nano Res. 2, 755–767 (2009). https://doi.org/10.1007/s12274-009-9078-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-009-9078-8