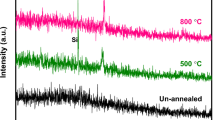
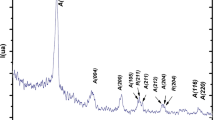
Abstract
TiS3 nanobelt films, with widths of about 0.1–12 μm, thickness of about 20–250 nm, and lengths of up to 200 μm, have been grown on Ti substrates by a surface-assisted chemical-vapor-transport at 450 °C for 8 h. The TiS3 nanobelt films were converted into TiS1.71 nanobelt films by pyrolysis in a vacuum at 600 °C for 2 h. The work functions of the two films were determined by ultraviolet photoelectron spectroscopy measurements to be 4.60 and 4.44 eV, respectively. Preliminary field emission experiments using the nanostructures as cold electron cathodes showed that both materials gave significant emission currents. The turn-on fields (defined as the electric field required to produce a current density of 10 μA/cm2) were about 1.0 and 0.9 V/μm, respectively, whereas the threshold fields (defined as the electric field required to produce a current density of 1 mA/cm2) were about 5.6 and 4.0 V/μm, respectively. These data reveal that both materials have potential applications in field emission devices.

Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Bawendi, M. G.; Steigerwald, M. L.; Brus, L. E. The quantum-mechanics of larger semiconductor clusters (quantum dots). Annu. Rev. Phys. Chem. 1990, 41, 477–496.
Alivisatos, A. P. Semiconductor clusters, nanocrystals, and quantum dots. Science 1996, 271, 933–937.
Weller, H. Colloidal semiconductor Q-particles—Chemistry in the transition region between solid-state and molecules. Angew. Chem. Int. Ed. 1993, 32, 41–53.
Xia, Y.; Yang, P.; Sun, Y.; Wu, Y.; Mayers, B.; Gates, B.; Yin, Y.; Kim, F.; Yan, H. One-dimensional nanostructures: Synthesis, characterization, and applications. Adv. Mater. 2003, 15, 353–389.
Kovtyukhova, N. I.; Mallouk, T. E. Nanowires as building blocks for self-assembling logic and memory circuits. Chem. Eur. J. 2002, 8, 4355–4363.
Bavykin, D. V.; Friedrich, J. M.; Walsh, F. C. Protonated titanates and TiO2 nanostructured materials: Synthesis, properties, and applications. Adv. Mater. 2006, 18, 2807–2824.
Wu, X. C.; Tao, Y. R.; Hu, Y. M.; Song, Y.; Hu, Z.; Zhu, J. J.; Dong, L. Tantalum disulfide nanobelts: Preparation, superconductivity and field emission. Nanotechnology 2006, 17, 201–205.
Fang, X.; Bando, Y.; Ye, C.; Golberg, D. Crystal orientation-ordered ZnS nanobelt quasi-arrays and their enhanced field-emission. Chem. Commun. 2007, 3048–3050.
Yoon, H.; Seo, K.; Moon, H.; Varadwaj, K. S. K.; In, J.; Kim, B. Aluminum foil mediated noncatalytic growth of ZnO nanowire arrays on an indium tin oxide substrate. J. Phys. Chem. C 2008, 112, 9181–9185.
Li, L.; Fang, X.; Chew, H. G.; Zheng, F.; Liew, T. H.; Xu, X.; Zhang, Y.; Pan, S.; Li, G.; Zhang, L. Crystallinity-controlled germanium nanowire arrays: Potential field emitters. Adv. Funct. Mater. 2008, 18, 1080–1088.
Ait-Ouali, A.; Jandl, S. Photoluminescence study of the one-dimensional material ZrS3 and its solid solution Zr1−xHfxS3. Phys. Rev. B 1996, 53, 9852–9858.
Shepherd, F. R.; Williams, P. M. Photoemission studies of band structures of transition-metal dichalcogenides. 1. Group-IVA and Group-IVB. J. Phys. C: Solid State Phys. 1974, 7, 4416–4426.
Chen, C. H.; Fabian, W.; Brown, F. C.; Woo, K. C.; Davies, B.; De Long, B.; Thompson, A. H. Angle-resolved photoemission-studies of the band-structure of TiSe2 and TiS2. Phys. Rev. B. 1980, 21, 615–624.
Chang, H. S. W.; Schleich, D. M. TiS2 and TiS3 thin-films prepared by MocVD. J. Solid State Chem. 1992, 100, 62–70.
Ma, J. J.; Liu, X.Y.; Cao, X. J.; Feng, S. H.; Fleet, M. E. Bundle of nanobelts up to 4 cm in length: One-step synthesis and preparation of titanium trisulfide (TiS3) nanomaterials. Eur. J. Inorg. Chem. 2006, 519–522.
Nath, M.; Rao, C. N. R. Nanotubes of group 4 metal disulfides. Angew. Chem. 2002, 41, 3451–3454.
Chen, J.; Tao, Z. L.; Li, S. L. Lithium intercalation in open-ended TiS2 nanotubes. Angew. Chem. Int. Ed. 2003, 42, 2147–2151.
Zhang, Y.; Li, Z. K.; Jia, H. B.; Luo, X. H.; Xu, J.; Zhang, X. H.; Yu, D. P. TiS2 whisker growth by a simple vapor-deposition meth. J. Cryst. Growth 2006, 293, 124–127.
Tao, Y. R.; Wu, X. C.; Zhang, Y. L.; Dong, L.; Zhu, J. J.; Hu, Z. Surface-assisted synthesis of microscale hexagonal plates and flower-like patterns of single-crystalline titanium disulfide and their field-emission properties. Cryst. Growth Des. 2008, 8, 2990–2994.
Jennings, H. M. On reactions between silicon and nitrogen. 1. Mechanisms. J. Mater. Sci. 1983, 18, 951–967.
Zhou, J.; Xu, N. S.; Deng, S. Z.; Chen, J.; She, J. C.; Wang, Z. L. Large-area nanowire arrays of molybdenum and molybdenum oxides: Synthesis and field emission properties. Adv. Mater. 2003, 15, 1835–1840.
Park, Y.; Choong, V.; Gao, Y.; Hsieh, B. R.; Tang, C. W. Work function of indium tin oxide transparent conductor measured by photoelectron spectroscopy. Appl. Phys. Lett. 1996, 68, 2699–2701.
Gadzuk, J. W.; Plummer, E. W. Field-emission energy-distribution (FEED). Rev. Mod. Phys. 1973, 45, 487 548.
Xu, N. S.; Chen, Y.; Deng, S. Z.; Chen, J.; Ma, X. C.; Wang, E. G. Vacuum gap dependence of field electron emission properties of large area multi-walled carbon nanotube films. J. Phys. D: Appl. Phys. 2001, 34, 1597–1601.
He, J. H.; Yang, R.; Chueh, Y. L.; Chou, L. J.; Chen, L. J.; Wang, Z. L. Aligned AlN nanorods with multi-tipped surfaces—Growth, field-emission, and cathodoluminescence properties. Adv. Mater. 2006, 18, 650–654.
Zhang, Y. L.; Wu, X. C.; Tao, Y. R.; Mao, C. J.; Zhu, J. J. Fabrication and field-emission performance of zirconium disulfide nanobelt arrays. Chem. Comm. 2008, 2683–2685.
Bai, X.; Wang, E. G.; Gao, P.; Wang, Z. L. Measuring the work function at a nanobelt tip and at a nanoparticle surface. Nano Lett. 2003, 3, 1147–1150.
Xu, Z.; Bai, X. D.; Wang, E. G.; Wang, Z. L. Field emission of individual carbon nanotube with in situ tip image and real work function. Appl. Phys. Lett. 2005, 87, 163–106.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published with open access at Springerlink.com
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Wu, X.C., Tao, Y.R. & Gao, Q.X. Preparation and field emission properties of titanium polysulfide nanobelt films. Nano Res. 2, 558–564 (2009). https://doi.org/10.1007/s12274-009-9055-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-009-9055-2