Abstract
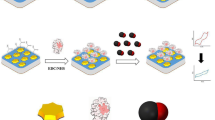
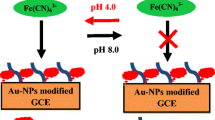
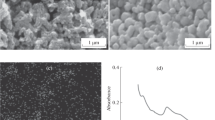
A novel biosensor based on a myoglobin/gold nanoparticles/carbon spheres (Mb-AuNPs-CNs) 3-D architecture bioconjunction has been fabricated for the determination of hydrogen peroxide (H2O2). Cyclic voltammetry (CV), Fourier transform infrared (FT-IR) spectroscopy and scanning electron microscopy (SEM) were used to characterize the bioconjunction of the AuNPs-CNs with Mb. Experimental results demonstrate that the AuNPs-CNs hybrid material is more effective in facilitating electron transfer of the immobilized enzyme than CNs alone, which can be attributed to the unique nanostructure and larger surface area of the bioconjunction. The biosensor displayed good performance for the detection of H2O2 with a wide linear range from 0.28 μmol/L to 116.5 μmol/L and a detection limit of 0.12 μmol/L. The Michaelis-Menten constant K appM value was estimated to be 0.3 mmol/L. The resulting biosensor exhibited fast amperometric response, and good stability, reproducibility, and selectivity to H2O2.

Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Rusling, J. F.; Nassar, A. E. F. Enhanced electron-transfer for myglobin in surfactant films on electrodes. J. Am. Chem. Soc. 1993, 115, 11891–11897.
Nassar, A. E. F.; Bobbitt, J. M.; Stuart, J. D.; Rusling, J. F. Catalytic reduction of organohalide pollutants by myoglobin in a biomembrane-like surfactant film. J. Am. Chem. Soc. 1995, 117, 10986–10993.
Zhao, G. C.; Zhang, L.; Wei, X. W.; Yang, Z. S. Myoglobin on multi-walled carbon nanotubes modified electrode: Direct electrochemistry and electrocatalysis. Electrochem. Commun. 2003, 5, 825–829.
Cai, C. X.; Chen, J. Direct electron transfer and bioelectrocatalysis of hemoglobin at a carbon nanotube electrode. Anal. Biochem. 2004, 325, 285–292.
Zhou, H. H.; Chen, H.; Luo, S. L.; Chen, J. H.; Wei, W. Z.; Kuang, Y. F. Glucose biosensor based on platinum microparticles dispersed in nano-fibrous polyaniline. Biosens. Bioelectron. 2005, 20, 1305–1311.
Zhang, L.; Jiang, X.; Niu, L.; Dong, S. J. Syntheses of fully sulfonated polyaniline nano-networks and its application to the direct electrochemistry of cytochrome c. Biosens. Bioelectron. 2006, 21, 1107–1115.
Zhang, L.; Zhang, Q.; Li, J. H. Direct electrochemistry and electrocatalysis of hemoglobin immobilized in bimodal mesoporous silica and chitosan inorganic-organic hybrid film. Electrochem. Commun. 2007, 9, 1530–1535.
Liu, X. Q.; Shi, L. H.; Niu, W. X.; Li, H. J.; Xu, G. B. Amperometric glucose biosensor based on single-walled carbon nanohorns. Biosens. Bioelectron. 2008, 23, 1887–1890.
Sotiropoulou, S.; Chaniotakis, N. A. Carbon nanotube array-based biosensor. Anal. Bioanal. Chem. 2003, 375, 103–105.
Banks, C. E.; Compton, R. G. Exploring the electrocatalytic sites of carbon nanotubes for NADH detection: An edge plane pyrolytic graphite electrode study. Analyst 2005, 130, 1232–1239.
Wang, J. Stripping analysis at bismuth electrodes: A review. Electroanalysis 2005, 17, 1341–1346.
Vamvakaki, V.; Tsagaraki, K.; Chaniotakis, N. Carbon nanofiber-based glucose biosensor. Anal. Chem. 2006, 78, 5538–5542.
Lu, X. B.; Zhou, J. H.; Lu, W.; Liu, Q.; Li, J. H. Carbon nanofiber-based composites for the construction of mediator free biosensors. Biosens. Bioelectron. 2008, 23, 1236–1243.
Sun, X. M.; Li, Y. D. Colloidal carbon spheres and their core/shell structures with noble-metal nanoparticles. Angew. Chem. 2004, 116, 607–611.
Balasubramanian, K.; Burghard, M. Electrochemically functionalized carbon nanotubes for device applications. Small 2005, 1, 180–192.
Cui, R. J.; Liu, C.; Shen, J. M.; Gao, D.; Zhu, J. J.; Chen, H. Y. Gold nanoparticle-colloidal carbon nanosphere hybrid material: Preparation, characterization, and application for an amplified electrochemical immunoassay. Adv. Funct. Mater. 2008, 18, 2197–2204.
Stargardt, J. F.; Hawkridge, F. M.; Landrum, H. L. Reversible heterogenous reduction and oxidation of sperm whale myoglobin at a surface modified gold minigrid electrode. Anal. Chem. 1978, 50, 930–932.
Hildebrand, D. P.; Tang, H. L.; Luo, Y. G.; Hunter, C. L.; Smith, M.; Brayer, G. D.; Mauk, A. G. Efficient coupled oxidation of heme by an active site variant of horse heart myoglobin. J. Am. Chem. Soc. 1996, 118, 12909–12915.
Yabuki, S.; Shinohara, H.; Aizawa, M. Electro-conductive enzyme membrane. J. Chem. Soc. Chem. Commun. 1989, 945–946.
Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. 1979, 101, 19–28.
Kendrew, J. C.; Bodo, G.; Dintzis, H. M.; Parrish, R. G.; Wyckoff, H.; Phillips, D. C. A three-dimensional model of the myoglobin molecule obtained by X-ray analysis. Nature 1958, 181, 622–666.
Leitch, F. A.; Moore, G. R.; Pettigrew, G. W. Structural basis for the variation of pH-dependent redox potentials of Pseudomonas cytochromes C-551. Biochem. 1984, 23, 1831–1838.
Wyman, J. Regulation in macromolecules, as illustrated by hemoglobin. Quart. Rev. Biophys. 1968, 35–80.
Bond, A. M. Modern Polarographic Methods in Analytical Chemistry. M. Dekker: New York, 1980, pp. 27–45.
Yamazaki, I.; Araiso, T.; Hayashi, Y.; Yamada, H.; Makino, R. Analysis of acid-base properties of peroxidase and myoglobin. Adv. Biophys. 1978, 11, 249–281.
Tatsuma, T.; Mori, H.; Fujishima, A. Electron transfer from diamond electrodes to heme peptide and peroxidase. Anal. Chem. 2000, 72, 2919–2924.
Kamin, R. A.; Wilson, G. S. Rotating-ring-disk enzyme electrode for biocatalysis kinetic-studies and characterization of the immobilized enzyme layer. Anal. Chem. 1980, 52, 1198–1205.
Zhao, Y. D.; Bi, Y. H.; Zhang, W. D.; Luo, Q. M. The interface behavior of hemoglobin at carbon nanotube and the detection for H2O2. Talanta 2005, 65, 489–494.
Zhao, X. J.; Mai, Z. B.; Kang, X. H.; Dai, Z.; Zou, X.Y. Clay-chitosan-gold nanoparticle nanohybrid: Preparation and application for assembly and direct electrochemistry of myoglobin. Electrochim. Acta 2008, 53, 4732–4739.
Zong, S. Z.; Cao, Y.; Zhou, Y. M.; Ju, H. X. Reagentless biosensor for hydrogen peroxide based on immobilization of protein in zirconia nanoparticles enhanced grafted collagen matrix. Biosens. Bioelectron. 2007, 22, 1776–1782.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published with open access at Springerlink.com
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Chen, X., Zhang, J.J., Xuan, J. et al. Myoglobin/gold nanoparticles/carbon spheres 3-D architecture for the fabrication of a novel biosensor. Nano Res. 2, 210–219 (2009). https://doi.org/10.1007/s12274-009-9019-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-009-9019-6