Abstract
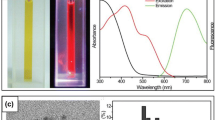
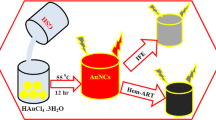
Two fluorescent quantum clusters of gold, namely Au25 and Au8, have been synthesized from mercaptosuccinic acid-protected gold nanoparticles of 4–5 nm core diameter by etching with excess glutathione. While etching at pH ∼3 yielded Au25, that at pH 7–8 yielded Au8. This is the first report of the synthesis of two quantum clusters starting from a single precursor. This simple method makes it possible to synthesize well-defined clusters in gram quantities. Since these clusters are highly fluorescent and are highly biocompatible due to their low metallic content, they can be used for diagnostic applications.

Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Balamurugan, B.; Maruyama, T. Evidence of an enhanced interband absorption in Au nanoparticles: Size-dependent electronic structure and optical properties. Appl. Phys. Lett. 2005, 87, 143105.
Jain, P. K.; Huang, X.; El-Sayed, I. H.; El-Sayed, M. A. Noble metals on the nanoscale: Optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc. Chem. Res. 2008, in press, [DOI: 10.1021/ar7002804].
Guo, R.; Song, Y.; Wang, G.; Murray, R. W. Does core size matter in the kinetics of ligand exchanges of monolayer-protected Au clusters? J. Am. Chem. Soc. 2005, 127, 2752–2757.
Duan, C.; Cui, H.; Zhang, Z.; Liu, B.; Guo, J.; Wang, W. Size-dependent inhibition and enhancement by gold nanoparticles of luminol-ferricyanide chemiluminescence. J. Phys. Chem. C 2007, 111, 4561–4566.
Ramakrishna, G.; Ghosh, H. N. Effect of particle size on the reactivity of quantum size ZnO nanoparticles and charge-transfer dynamics with adsorbed catechols. Langmuir 2003, 19, 3006–3012.
Zheng, J.; Nicovich, P. R.; Dickson, R. M. Highly fluorescent noble-metal quantum dots. Annu. Rev. Phys. Chem. 2007, 58, 409–431.
Zheng, J.; Petty, J. T.; Dickson, R. M. High quantum yield blue emission from water-soluble Au8 nanodots. J. Am. Chem. Soc. 2003, 125, 7780–7781.
Zheng, J.; Zhang, C. W.; Dickson, R. M. Highly fluorescent, water-soluble, size-tunable gold quantum dots. Phys. Rev. Lett. 2004, 93, 077402.
Duan, H.; Nie, S. Etching colloidal gold nanocrystals with hyperbranched and multivalent polymers: A new route to fluorescent and water-soluble atomic clusters. J Am. Chem. Soc. 2007, 129, 2412–2413.
Woehrle, G. H.; Hutchison, J. E. Thiol-functionalized undecagold clusters by ligand exchange: Synthesis, mechanism, and properties. Inorg. Chem. 2005, 44, 6149–6158.
Bertino, M. F.; Sun, Z.-M.; Zhang, R.; Wang, L.-S. Facile syntheses of monodisperse ultrasmall Au clusters. J. Phys. Chem. B 2006, 110, 21416–21418.
Nunokawa, K.; Onaka, S.; Ito, M.; Horibe, M.; Yonezawa, T.; Nishihara, H.; Ozeki, T.; Chiba, H.; Watase, S.; Nakamoto, M. Synthesis, single crystal X-ray analysis, and TEM for a single-sized Au11 cluster stabilized by SR ligands: The interface between molecules and particles. J. Organomet. Chem. 2006, 691, 638–642.
Yanagimoto, Y.; Negishi, Y.; Fujihara, H.; Tsukuda, T. Chiroptical activity of BINAP-stabilized undecagold clusters. J. Phys. Chem. B 2006, 110, 11611–11614.
Woehrle, G. H.; Warner, M. G.; Hutchison, J. E. Ligand exchange reactions yield subnanometer, thiol-stabilized gold particles with defined optical transitions. J. Phys. Chem. B 2002, 106, 9979–9981.
Menard, L. D.; Gao, S.-P.; Xu, H.; Twesten, R. D.; Harper, A. S.; Song, Y.; Wang, G.; Douglas, A. D.; Yang, J. C.; Frenkel, A. I. et al. Sub-nanometer Au monolayer-protected clusters exhibiting molecule-like electronic behavior: Quantitative high-angle annular dark-field scanning transmission electron microscopy and electrochemical characterization of clusters with precise atomic stoichiometry. J. Phys. Chem. B 2006, 110, 12874–12883.
Abad, J. M.; Sendroiu, I. E.; Gass, M.; Bleloch, A.; Mills, A. J.; Schiffrin D. J. Synthesis of ω-hydroxy hexathiolate-protected subnanometric gold clusters. J. Am. Chem. Soc. 2007, 129, 12932–12933.
Menard, L. D.; Xu, H.; Gao, S. P.; Twesten, R. D.; Harper, A. S.; Song, Y.; Wang, G.; Douglas, A. D.; Yang, J. C.; Frenkel, A. I.; et al. Metal core bonding motifs of monodisperse icosahedral Au13 and larger Au monolayer-protected clusters as revealed by X-ray absorption spectroscopy and transmission electron microscopy. J. Phys. Chem. B 2006, 110, 14564–14573.
Schaaff, T. G.; Knight, G.; Shafigullin, M. N.; Borkman, R. F.; Whetten, R. L. Isolation and selected properties of a 10.4 kDa gold: Glutathione cluster compound. J. Phys. Chem. B 1998, 102, 10643–10646.
Negishi, Y.; Nobusada, K.; Tsukuda, T. Glutathione-protected gold clusters revisited: Bridging the gap between gold(I)-thiolate complexes and thiolate-protected gold nanocrystals. J. Am. Chem. Soc. 2005, 127, 5261–5270.
Shichibu, Y.; Negishi, Y.; Tsukuda, T.; Teranishi, T. Large-scale synthesis of thiolated Au25 clusters via ligand exchange reactions of phosphine-stabilized Au11 clusters. J. Am. Chem. Soc. 2005, 127, 13464–13465.
Shichibu, Y.; Negishi, Y.; Tsunoyama, H.; Kanehara, M.; Teranishi, T.; Tsukuda T. Extremely high stability of glutathionate-protected Au25 clusters against core etching. Small 2007, 3, 835–839.
Habeeb Muhammed, M. A.; Pradeep, T. Reactivity of Au25 clusters with Au3+. Chem. Phys. Lett. 2007, 449, 186–190.
Shibu, E. S.; Habeeb Muhammed, M. A.; Tsukuda, T.; Pradeep, T. Ligand exchange of Au25SG18 leading to functionalized gold clusters: spectroscopy, kinetics, and luminescence. J. Phys. Chem C 2008, 112, 12168–12176.
Habeeb Muhammed, M. A.; Shaw, A. K.; Pal, S. K.; Pradeep T. Quantum clusters of gold exhibiting FRET. J. Phys. Chem C, 2008, 112, 14324–14330.
Zhu, M.; Lanni, E.; Garg, N.; Bier, M. E.; Jin, R. Kinetically controlled, high-yield synthesis of Au25 clusters. J. Am. Chem. Soc. 2008, 130, 1138–1139.
Lee, T.-H.; Gonzalez, J. I.; Zheng, J.; Dickson, R. M. Single-molecule optoelectronics. Acc. Chem. Res. 2005, 38, 534–541.
Zhu, M.; Aikens, C. M.; Hollander, F. J.; Schatz, G. C.; Jin, R. Correlating the crystal structure of a thiol-protected Au25 cluster and optical properties. J. Am. Chem. Soc. 2008, 130, 5883–5885.
Huang, T.; Murray, R. W. Visible luminescence of water-soluble monolayer-protected gold clusters. J. Phys. Chem. B 2001, 105, 12498–12502.
Brinas, R. P.; Hu, M.; Qian, L.; Lymar, E. S.; Hainfeld, J. F. Gold nanoparticle size controlled by polymeric Au(I) thiolate precursor size. J. Am. Chem. Soc. 2008, 130, 975–982.
Nishida, N.; Shibu, E. S.; Yao, H.; Oonishi, T.; Kimura, K.; Pradeep, T. Fluorescent gold nanoparticle superlattices. Adv. Mater. 2008, in press, [DOI: 10.1002/adma.200800632].
Chen, S.; Kimura, K. Synthesis and characterization of carboxylate-modified gold nanoparticle powders dispersible in water. Langmuir 1999, 15, 1075–1082.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published with open access at Springerlink.com
Electronic supplementary material
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Habeeb Muhammed, M.A., Ramesh, S., Sinha, S.S. et al. Two distinct fluorescent quantum clusters of gold starting from metallic nanoparticles by pH-dependent ligand etching. Nano Res. 1, 333–340 (2008). https://doi.org/10.1007/s12274-008-8035-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-008-8035-2