Abstract
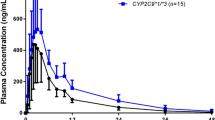
Tamsulosin, a selective \({\upalpha }_{1}\)-adrenoceptor blocker, is commonly used for alleviation of lower urinary tract symptoms related to benign prostatic hyperplasia. Tamsulosin is predominantly metabolized by CYP3A4 and CYP2D6 enzymes, and several studies reported the effects of CYP2D6 genetic polymorphism on the pharmacokinetics of tamsulosin. This study aims to develop and validate the physiologically based pharmacokinetic (PBPK) model of tamsulosin in CYP2D6*wt/*wt, CYP2D6*wt/*10, and CYP2D6*10/*10 genotypes, using Simcyp® simulator. Physicochemical, and formulation properties and data for absorption, distribution, metabolism and excretion were collected from previous publications, predicted in the simulator, or optimized in different CYP2D6 genotypes. The tamsulosin PBPK model in CYP2D6*wt/*wt and CYP2D6*wt/*10 genotypes were developed based on the clinical pharmacokinetic study where a single oral dose of 0.2 mg tamsulosin was administered to 25 healthy Korean male volunteers with CYP2D6*wt/*wt and CYP2D6*wt/*10 genotypes. A previous pharmacokinetic study was used to develop the model in CYP2D6*10/*10 genotype. The developed model was validated using other clinical pharmacokinetic studies not used in development. The predicted exposures via the PBPK model in CYP2D6*wt/*10 and CYP2D6*10/*10 genotype was 1.23- and 1.76-fold higher than CYP2D6*wt/*wt genotype, respectively. The simulation profiles were visually similar to the observed profiles, and fold errors of all development and validation datasets were included within the criteria. Therefore, the tamsulosin PBPK model in different CYP2D6 genotypes with regards to CYP2D6*10 alleles was appropriately established. Our model can contribute to the implementation of personalized pharmacotherapy of patients, appropriately predicting the pharmacokinetics of tamsulosin reflecting their demographic and CYP2D6 genotype characteristics without unnecessary drug exposure.




Similar content being viewed by others
References
Ashley C, Dunleavy A, Cunningham J (2018) The renal drug handbook: the ultimate prescribing guide for renal practitioners, 5th edn. CRC Press, Boca Raton, p 960. https://doi.org/10.1201/9780429460418
Bae JW, Oh KY, Yoon SJ, Shin HB, Jung EH, Cho CK, Lim CW, Kang P, Choi CI, Jang CG, Lee SY, Lee YJ (2020) Effects of CYP2D6 genetic polymorphism on the pharmacokinetics of metoclopramide. Arch Pharm Res 43(11):1207–1213. https://doi.org/10.1007/s12272-020-01293-4
Ban MS, Kim YK, Kim B, Jung J, Kim YI, Oh J, Yu KS (2020) Evaluation of the pharmacokinetics and food effects of a novel formulation tamsulosin 0.4 mg capsule compared with a 0.2 mg capsule in healthy male volunteers. Transl Clin Pharmacol 28(4):181–188. https://doi.org/10.12793/tcp.2020.28.e17
Berezhkovskiy LM (2004) Volume of distribution at steady state for a linear pharmacokinetic system with peripheral elimination. J Pharm Sci 93(6):1628–1640. https://doi.org/10.1002/jps.20073
Birmingham BK, Bujac SR, Elsby R, Azumaya CT, Zalikowski J, Chen Y, Kim K, Ambrose HJ (2015) Rosuvastatin pharmacokinetics and pharmacogenetics in Caucasian and Asian subjects residing in the United States. Eur J Clin Pharmacol 71(3):329–340. https://doi.org/10.1007/s00228-014-1800-0
Boehringer Ingelheim (2009) Flomax® (tamsulosin hydrochloride) prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020579s025lbl.pdf. Accessed 13 Oct 2021
Bowman CM, Benet LZ (2019) Interlaboratory variability in human hepatocyte intrinsic clearance values and trends with physicochemical properties. Pharm Res 36(8):113. https://doi.org/10.1007/s11095-019-2645-0
Byeon JY, Kim YH, Na HS, Jang JH, Kim SH, Lee YJ, Bae JW, Kim IS, Jang CG, Chung MW, Lee SY (2015) Effects of the CYP2D6*10 allele on the pharmacokinetics of atomoxetine and its metabolites. Arch Pharm Res 38(11):2083–2091. https://doi.org/10.1007/s12272-015-0646-z
Byeon JY, Kim YH, Lee CM, Kim SH, Chae WK, Jung EH, Choi CI, Jang CG, Lee SY, Bae JW, Lee YJ (2018a) CYP2D6 allele frequencies in Korean population, comparison with East Asian, Caucasian and African populations, and the comparison of metabolic activity of CYP2D6 genotypes. Arch Pharm Res 41(9):921–930. https://doi.org/10.1007/s12272-018-1075-6
Byeon JY, Lee YJ, Kim YH, Kim SH, Lee CM, Bae JW, Jang CG, Lee SY, Choi CI (2018b) Effects of diltiazem, a moderate inhibitor of CYP3A4, on the pharmacokinetics of tamsulosin in different CYP2D6 genotypes. Arch Pharm Res 41(5):564–570. https://doi.org/10.1007/s12272-018-1030-6
Byeon JY, Lee CM, Lee YJ, Kim YH, Kim SH, Jung EH, Chae WK, Lee YJ, Jang CG, Lee SY (2019) Influence of CYP2D6 genetic polymorphism on pharmacokinetics of active moiety of tolterodine. Arch Pharm Res 42(2):182–190. https://doi.org/10.1007/s12272-018-1099-y
Choi CI, Bae JW, Jang CG, Lee SY (2012a) Tamsulosin exposure is significantly increased by the CYP2D6*10/*10 genotype. J Clin Pharmacol 52(12):1934–1938. https://doi.org/10.1177/0091270011432168
Choi CI, Lee HI, Bae JW, Lee YJ, Byeon JY, Jang CG, Lee SY (2012b) Determination of tamsulosin in human plasma by liquid chromatography/tandem mass spectrometry and its application to a pharmacokinetic study. J Chromatogr B 909:65–69. https://doi.org/10.1016/j.jchromb.2012.10.012
Chu N, Xu H, Wang G, Wang J, Chen W, Yuan F, Yang M, Li X (2015) Pharmacokinetic interaction of finasteride with tamsulosin hydrochloride: an open-label, randomized, 3-period crossover study in healthy Chinese male volunteers. Clin Ther 37(2):462–472. https://doi.org/10.1016/j.clinthera.2014.10.021
Djebli N, Fabre D, Boulenc X, Fabre G, Sultan E, Hurbin F (2015) Physiologically based pharmacokinetic modeling for sequential metabolism: effect of CYP2C19 genetic polymorphism on clopidogrel and clopidogrel active metabolite pharmacokinetics. Drug Metab Dispos 43(4):510–522. https://doi.org/10.1124/dmd.114.062596
Duan P, Zhao P, Zhang L (2017) Physiologically Based Pharmacokinetic (PBPK) modeling of pitavastatin and atorvastatin to predict Drug-Drug Interactions (DDIs). Eur J Drug Metab Pharmacokinet 42(4):689–705. https://doi.org/10.1007/s13318-016-0383-9
European Medicines Agency (2018) Guideline on the reporting of physiologically based pharmacokinetic (PBPK) modeling and simulation. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-reporting-physiologically-based-pharmacokinetic-pbpk-modelling-simulation_en.pdf. Accessed 13 Oct 2021
Food and Drug Administration (2018) Physiologically Based Pharmacokinetic Analyses—Format and Content Guidance for Industry. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM531207.pdf. Accessed 13 Oct 2021
Fossler MJ, Collins DA, Thompson MM, Nino A, Bianco JJ, Chetty D (2014) Pharmacokinetic bioequivalence studies of a fixed-dose combination of tamsulosin and dutasteride in healthy volunteers. Clin Drug Investig 34(5):335–349. https://doi.org/10.1007/s40261-014-0179-0
Franco-Salinas G, de la Rosette JJ, Michel MC (2010) Pharmacokinetics and pharmacodynamics of tamsulosin in its modified-release and oral controlled absorption system formulations. Clin Pharmacokinet 49(3):177–188. https://doi.org/10.2165/11317580-000000000-00000
Fukunaga S, Kusama M, Arnold FL, Ono S (2011) Ethnic differences in pharmacokinetics in new drug applications and approved doses in Japan. J Clin Pharmacol 51(8):1237–1240. https://doi.org/10.1177/0091270010381500
George B, Lumen A, Nguyen C, Wesley B, Wang J, Beitz J, Crentsil V (2020) Application of physiologically based pharmacokinetic modeling for sertraline dosing recommendations in pregnancy. NPJ Syst Biol Appl 6(1):36. https://doi.org/10.1038/s41540-020-00157-3
Huth F, Gardin A, Umehara K, He H (2019) Prediction of the impact of cytochrome P450 2C9 genotypes on the drug-drug interaction potential of siponimod with physiologically-based pharmacokinetic modeling: a comprehensive approach for drug label recommendations. Clin Pharmacol Ther 106(5):1113–1124. https://doi.org/10.1002/cpt.1547
Ingelman-Sundberg M (2005) Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): clinical consequences, evolutionary aspects and functional diversity. Pharmacogenomics 5(1):6–13. https://doi.org/10.1038/sj.tpj.6500285
Jamei M, Marciniak S, Feng K, Barnett A, Tucker G, Rostami-Hodjegan A (2009) The Simcyp population-based ADME simulator. Expert Opin Drug Metab Toxicol 5(2):211–223. https://doi.org/10.1517/17425250802691074
Johansson I, Oscarson M, Yue QY, Bertilsson L, Sjöqvist F, Ingelman-Sundberg M (1994) Genetic analysis of the Chinese cytochrome P4502D locus: characterization of variant CYP2D6 genes present in subjects with diminished capacity for debrisoquine hydroxylation. Mol Pharmacol 46(3):452–459
Johnson TN, Boussery K, Rowland-Yeo K, Tucker GT, Rostami-Hodjegan A (2010) A semi-mechanistic model to predict the effects of liver cirrhosis on drug clearance. Clin Pharmacokinet 49(3):189–206. https://doi.org/10.2165/11318160-000000000-00000
Jung EH, Lee CM, Byeon JY, Shin HB, Oh KY, Cho CK, Lim CW, Jang CG, Lee SY, Lee YJ (2020a) Relationship between plasma exposure of zolpidem and CYP2D6 genotype in healthy Korean subjects. Arch Pharm Res 43(9):976–981. https://doi.org/10.1007/s12272-020-01250-1
Jung EH, Lee YJ, Kim DH, Kang P, Lim CW, Cho CK, Jang CG, Lee SY, Bae JW (2020b) Effects of paroxetine on the pharmacokinetics of atomoxetine and its metabolites in different CYP2D6 genotypes. Arch Pharm Res 43(12):1356–1363. https://doi.org/10.1007/s12272-020-01300-8
Kim KA, Park IB, Park JY (2018a) Effects of CYP2D6 and CYP3A5 genetic polymorphisms on steady-state pharmacokinetics and hemodynamic effects of tamsulosin in humans. Eur J Clin Pharmacol 74(10):1281–1289. https://doi.org/10.1007/s00228-018-2501-x
Kim SH, Byeon JY, Kim YH, Lee CM, Lee YJ, Jang CG, Lee SY (2018b) Physiologically based pharmacokinetic modelling of atomoxetine with regard to CYP2D6 genotypes. Sci Rep 8(1):12405. https://doi.org/10.1038/s41598-018-30841-8
Kim Y, Hatley O, Rhee SJ, Yi S, Lee HA, Yoon S, Chung JY, Yu KS, Lee H (2019) Development of a Korean-specific virtual population for physiologically based pharmacokinetic modelling and simulation. Biopharm Drug Dispos 40(3–4):135–150. https://doi.org/10.1002/bdd.2178
Kim YH, Kang P, Cho CK, Jung EH, Park HJ, Lee YJ, Bae JW, Jang CG, Lee SY (2021) Physiologically based pharmacokinetic (PBPK) modeling for prediction of celecoxib pharmacokinetics according to CYP2C9 genetic polymorphism. Arch Pharm Res 44(7):713–724. https://doi.org/10.1007/s12272-021-01346-2
Kneller LA, Abad-Santos F, Hempel G (2020) Physiologically based pharmacokinetic modelling to describe the pharmacokinetics of risperidone and 9-hydroxyrisperidone according to cytochrome P450 2D6 phenotypes. Clin Pharmacokinet 59(1):51–65. https://doi.org/10.1007/s40262-019-00793-x
Koiso K, Akaza H, Kikuchi K, Aoyagi K, Ohba S, Miyazaki M, Ito M, Sueyoshi T, Matsushima H, Kamimura H, Watanabe T, Higuchi S (1996) Pharmacokinetics of tamsulosin hydrochloride in patients with renal impairment: effects of alpha 1-acid glycoprotein. J Clin Pharmacol 36(11):1029–1038. https://doi.org/10.1177/009127009603601107
Lepor H (1998a) Long-term evaluation of tamsulosin in benign prostatic hyperplasia: placebo-controlled, double-blind extension of phase III trial. Tamsulosin Investigator Group Urology 51(6):901–906. https://doi.org/10.1016/s0090-4295(98)00127-7
Lepor H (1998b) Phase III multicenter placebo-controlled study of tamsulosin in benign prostatic hyperplasia. Tamsulosin Investig Group Urol 51(6):892–900. https://doi.org/10.1016/s0090-4295(98)00126-5
Li X, Frechen S, Moj D, Lehr T, Taubert M, Hsin CH, Mikus G, Neuvonen PJ, Olkkola KT, Saari TI, Fuhr U (2020) A physiologically based pharmacokinetic model of voriconazole integrating time-dependent inhibition of CYP3A4, genetic polymorphisms of CYP2C19 and predictions of drug-drug interactions. Clin Pharmacokinet 59(6):781–808. https://doi.org/10.1007/s40262-019-00856-z
Lee CM, Jung EH, Byeon JY, Kim SH, Jang CG, Lee YJ, Lee SY (2019) Effects of steady-state clarithromycin on the pharmacokinetics of zolpidem in healthy subjects. Arch Pharm Res 42(12):1101–1106. https://doi.org/10.1007/s12272-019-01201-5
Loisios-Konstantinidis I, Cristofoletti R, Jamei M, Turner D, Dressman J (2020) Physiologically based pharmacokinetic/pharmacodynamic modeling to predict the impact of CYP2C9 genetic polymorphisms, co-medication and formulation on the pharmacokinetics and pharmacodynamics of flurbiprofen. Pharmaceutics 12(11):1049. https://doi.org/10.3390/pharmaceutics12111049
Lu J, Yang Y, Lu J, Wang Z, He Y, Yan Y, Fu K, Jiang W, Xu Y, Wu R, Liu W, Zhao J (2021) Effect of CYP2D6 polymorphisms on plasma concentration and therapeutic effect of risperidone. BMC Psychiatry 21(1):70. https://doi.org/10.1186/s12888-020-03034-9
Luzon E, Blake K, Cole S, Nordmark A, Versantvoort C, Berglund EG (2017) Physiologically based pharmacokinetic modeling in regulatory decision-making at the European Medicines Agency. Clin Pharmacol Ther 102(1):98–105. https://doi.org/10.1002/cpt.539
Maeda A, Shinoda T, Ito N, Baba K, Oku N, Mizumoto T (2011) Evaluating tamsulosin hydrochloride-released microparticles prepared using single-step matrix coating. Int J Pharm 408(1–2):84–90. https://doi.org/10.1016/j.ijpharm.2011.01.053
Marsousi N, Desmeules JA, Rudaz S, Daali Y (2017) Usefulness of PBPK modeling in incorporation of clinical conditions in personalized medicine. J Pharm Sci 106(9):2380–2391. https://doi.org/10.1016/j.xphs.2017.04.035
Matsushima H, Kamimura H, Soeishi Y, Watanabe T, Higuchi S, Tsunoo M (1998) Pharmacokinetics and plasma protein binding of tamsulosin hydrochloride in rats, dogs, and humans. Drug Metab Dispos 26(3):240–245
Michel MC, Grübbel B, Taguchi K, Verfürth F, Otto T, Kröpfl D (1996) Drugs for treatment of benign prostatic hyperplasia: affinity comparison at cloned alpha 1-adrenoceptor subtypes and in human prostate. J Auton Pharmacol 16(1):21–28. https://doi.org/10.1111/j.1474-8673.1996.tb00352.x
Miyazawa Y, Blum RA, Schentag JJ, Kamimura H, Matsushima H, Swarz H, Ito Y (2001) Pharmacokinetics and safety of tamsulosin in subjects with normal and impaired renal or hepatic function. Curr Ther Res 62(9):603–621. https://doi.org/10.1016/S0011-393X(01)80067-9
Modi NB, Kell S, Aquilina J, Rivas D (2008) Effect of dapoxetine on the pharmacokinetics and hemodynamic effects of tamsulosin in men on a stable dose of tamsulosin. J Clin Pharmacol 48(12):1438–1450. https://doi.org/10.1177/0091270008324695
Narayan P, Tewari A (1998) A second phase III multicenter placebo controlled study of 2 dosages of modified release tamsulosin in patients with symptoms of benign prostatic hyperplasia, United States 93–01 Study Group. J Urol 160(5):1701–1706
Park SI, Rhee SJ, Jang IJ, Yu KS, Yim SV, Kim BH (2015) Bioequivalence of the pharmacokinetics between two formulations of 0.2mg tamsulosin hydrochloride in healthy subjects. Transl Clin Pharmacol 23(1):21–25. https://doi.org/10.12793/tcp.2015.23.1.21
Parmentier Y, Pothier C, Delmas A, Caradec F, Trancart MM, Guillet F, Bouaita B, Chesne C, Houston JB, Walther B (2017) Direct and quantitative evaluation of the human CYP3A4 contribution (fm) to drug clearance using the in vitro SILENSOMES model. Xenobiotica 47(7):562–575. https://doi.org/10.1080/00498254.2016.1208854
Price DT, Schwinn DA, Lomasney JW, Allen LF, Caron MG, Lefkowitz RJ (1993) Identification, quantification, and localization of mRNA for three distinct alpha 1 adrenergic receptor subtypes in human prostate. J Urol 150(2 Pt 1):546–551. https://doi.org/10.1016/s0022-5347(17)35544-1
Rodgers T, Rowland M (2006) Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci 95(6):1238–1257. https://doi.org/10.1002/jps.20502
Rodgers T, Leahy D, Rowland M (2005) Physiologically based pharmacokinetic modeling 1: predicting the tissue distribution of moderate-to-strong bases. J Pharm Sci 94(6):1259–1276. https://doi.org/10.1002/jps.20322
Rowland Yeo K, Aarabi M, Jamei M, Rostami-Hodjegan A (2011) Modeling and predicting drug pharmacokinetics in patients with renal impairment. Expert Rev Clin Pharmacol 4(2):261–274. https://doi.org/10.1586/ecp.10.143
Rüdesheim S, Wojtyniak JG, Selzer D, Hanke N, Mahfoud F, Schwab M, Lehr T (2020) Physiologically based pharmacokinetic modeling of metoprolol enantiomers and α-hydroxymetoprolol to describe CYP2D6 drug-gene interactions. Pharmaceutics 12(12):1200. https://doi.org/10.3390/pharmaceutics12121200
Sager JE, Yu J, Ragueneau-Majlessi I, Isoherranen N (2015) Physiologically Based Pharmacokinetic (PBPK) modeling and simulation approaches: a systematic review of published models, applications, and model verification. Drug Metab Dispos 43(11):1823–1837. https://doi.org/10.1124/dmd.115.065920
Seong SJ, Lee HW, Lee J, Lim MS, Kim EH, Park SM, Gwon MR, Yoon YR (2013) Steady-state pharmacokinetics properties of tamsulosin in healthy male volunteers. J Korean Soc Clin Pharmacol 21(2):130–140. https://doi.org/10.12793/jkscpt.2013.21.2.130
Shin HB, Jung EH, Kang P, Lim CW, Oh KY, Cho CK, Lee YJ, Choi CI, Jang CG, Lee SY, Bae JW (2020) ABCB1 c.2677G>T/c.3435C>T diplotype increases the early-phase oral absorption of losartan. Arch Pharm Res 43(11):1187–1196. https://doi.org/10.1007/s12272-020-01294-3
Soeishi Y, Matsushima H, Watanabe T, Higuchi S, Cornelissen K, Ward J (1996) Absorption, metabolism and excretion of tamsulosin hydrochloride in man. Xenobiotica 26(6):637–645. https://doi.org/10.3109/00498259609046739
Taguchi K, Schäfers RF, Michel MC (1998) Radioreceptor assay analysis of tamsulosin and terazosin pharmacokinetics. Br J Clin Pharmacol 45(1):49–55. https://doi.org/10.1046/j.1365-2125,1998.00636.x
Troost J, Tatami S, Tsuda Y, Mattheus M, Mehlburger L, Wein M, Michel MC (2011) Effects of strong CYP2D6 and 3A4 inhibitors, paroxetine and ketoconazole, on the pharmacokinetics and cardiovascular safety of tamsulosin. Br J Clin Pharmacol 72(2):247–256. https://doi.org/10.1111/j.1365-2125.2011.03988.x
Werk AN, Cascorbi I (2014) Functional gene variants of CYP3A4. Clin Pharmacol Ther 96(3):340–348. https://doi.org/10.1038/clpt.2014.129
Willmann S, Höhn K, Edginton A, Sevestre M, Solodenko J, Weiss W, Lippert J, Schmitt W (2007) Development of a physiology-based whole-body population model for assessing the influence of individual variability on the pharmacokinetics of drugs. J Pharmacokinet Pharmacodyn 34(3):401–431. https://doi.org/10.1007/s10928-007-9053-5
Wolzt M, Fabrizii V, Dorner GT, Zanaschka G, Leufkens P, Krauwinkel WJ, Eichler HG (1998) Pharmacokinetics of tamsulosin in subjects with normal and varying degrees of impaired renal function: an open-label single-dose and multiple-dose study. Eur J Clin Pharmacol 54(4):367–373. https://doi.org/10.1007/s002280050477
Xu M, Zheng L, Zeng J, Xu W, Jiang X, Wang L (2021) Physiologically based pharmacokinetic modeling of tramadol to inform dose adjustment and drug-drug interactions according to CYP2D6 phenotypes. Pharmacotherapy 41(3):277–290. https://doi.org/10.1002/phar.2494
Zhang X, Yang Y, Grimstein M, Fan J, Grillo JA, Huang SM, Zhu H, Wang Y (2020) Application of PBPK modeling and simulation for regulatory decision making and its impact on US prescribing information: an update on the 2018–2019 submissions to the US FDA’s Office of Clinical Pharmacology. J Clin Pharmacol 60(Suppl 1):S160–S178. https://doi.org/10.1002/jcph.1767
Zhao P, Rowland M, Huang SM (2012) Best practice in the use of physiologically based pharmacokinetic modeling and simulation to address clinical pharmacology regulatory questions. Clin Pharmacol Ther 92(1):17–20. https://doi.org/10.1038/clpt.2012.68
Zhuang X, Lu C (2016) PBPK modeling and simulation in drug research and development. Acta Pharm Sin B 6(5):430–440. https://doi.org/10.1016/j.apsb.2016.04.004
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future Planning (NRF-2019R1A2C1004582).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cho, C., Kang, P., Park, HJ. et al. Physiologically based pharmacokinetic (PBPK) modelling of tamsulosin related to CYP2D6*10 allele. Arch. Pharm. Res. 44, 1037–1049 (2021). https://doi.org/10.1007/s12272-021-01357-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-021-01357-z