Abstract
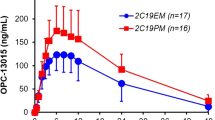
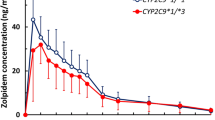
Zafirlukast, a cysteinyl leukotriene receptor antagonist, is indicated for the treatment of patients with mild to moderate asthma. Zafirlukast is metabolized mainly by CYP3A4 and CYP2C9. We investigated the effects of the major CYP2C9 variant alleles in Asian populations, CYP2C9*3 and CYP2C9*13, on the pharmacokinetics of zafirlukast in healthy Korean subjects. A single 20-mg oral dose of zafirlukast was given to 23 Korean male subjects divided into two genotype groups according to CYP2C9 genotypes, CYP2C9EM (n = 11; CYP2C9*1/*1) and CYP2C9IM (n = 12; 9 and 3 carriers of CYP2C9*1/*3 and *1/*13, respectively). Zafirlukast concentrations were determined using a validated HPLC–MS/MS analytical method in plasma samples collected after the drug intake. Compared with the CYP2C9EM group, the Cmax and AUCinf of zafirlukast in the CYP2C9IM group were 1.44- and 1.70-fold higher, respectively (p < 0.01 and p < 0.0001). The CL/F of zafirlukast was 42.8 % lower in the CYP2C9IM group compared with the CYP2C9EM group (p < 0.001). Slightly higher Cmax and AUC, and lower CL/F of zafirlukast were observed in subjects with the CYP2C9*1/*13 genotype compared with the CYP2C9*1/*3 genotype subjects. CYP2C9*3 and CYP2C9*13 alleles significantly affected the plasma concentrations of zafirlukast.


Similar content being viewed by others
References
Accolate label information (2013) AstraZeneca Pharmaceuticals LP, Wilmington, DE, USA.http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/020547s033lbl.pdf. Accessed 20 May 2016
Bae JW, Kim HK, Kim JH, Yang SI, Kim MJ, Jang CG, Park YS, Lee SY (2005) Allele and genotype frequencies of CYP2C9 in a Korean population. Br J Clin Pharmacol 60:418–422
Bae JW, Choi CI, Jang CG, Lee SY (2011a) Effects of CYP2C9*1/*13 on the pharmacokinetics and pharmacodynamics of meloxicam. Br J Clin Pharmacol 71:550–555
Bae JW, Choi CI, Kim MJ, Oh DH, Keum SK, Park JI, Kim BH, Bang HK, Oh SG, Kang BS, Park HJ, Kim HD, Ha JH, Shin HJ, Kim YH, Na HS, Chung MW, Jang CG, Lee SY (2011b) Frequency of CYP2C9 alleles in Koreans and their effects on losartan pharmacokinetics. Acta Pharmacol Sin 32:1303–1308
Bae JW, Choi CI, Lee HI, Lee YJ, Jang CG, Lee SY (2012) Effects of CYP2C9*1/*3 and *1/*13 on the pharmacokinetics of losartan and its active metabolite E-3174. Int J Clin Pharmacol Ther 50:683–689
Calhoun WJ (1998) Summary of clinical trials with zafirlukast. Am J Respir Crit Care Med 157:S238–S246
Choi CI, Kim MJ, Jang CG, Park YS, Bae JW, Lee SY (2011) Effects of the CYP2C9*1/*13 genotype on the pharmacokinetics of lornoxicam. Basic Clin Pharmacol Toxicol 109:476–480
Choi CI, Kim MJ, Chung EK, Lee HI, Jang CG, Bae JW, Lee SY (2012) CYP2C9*3 and *13 alleles significantly affect the pharmacokinetics of irbesartan in healthy Korean subjects. Eur J Clin Pharmacol 68:149–154
CYP2C9 allele nomenclature (2016) The Human Cytochrome P450 (CYP) Allele Nomenclature Committee. http://www.cypalleles.ki.se/cyp2c9.htm. Accessed 20 May 2016
Dupont WD, Plummer WD Jr (1998) Power and sample size calculations for studies involving linear regression. Control Clin Trials 19:589–601
García-Martín E, Martínez C, Ladero JM, Agúndez JA (2006) Interethnic and intraethnic variability of CYP2C8 and CYP2C9 polymorphisms in healthy individuals. Mol Diagn Ther 10:29–40
Guo Y, Zhang Y, Wang Y, Chen X, Si D, Zhong D, Fawcett JP, Zhou H (2005a) Role of CYP2C9 and its variants (CYP2C9*3 and CYP2C9*13) in the metabolism of lornoxicam in humans. Drug Metab Dispos 33:749–753
Guo Y, Wang Y, Si D, Fawcett PJ, Zhong D, Zhou H (2005b) Catalytic activities of human cytochrome P450 2C9*1, 2C9*3 and 2C9*13. Xenobiotica 35:853–861
Karonen T, Filppula A, Laitila J, Niemi M, Neuvonen PJ, Backman JT (2010) Gemfibrozil markedly increases the plasma concentrations of montelukast: a previously unrecognized role for CYP2C8 in the metabolism of montelukast. Clin Pharmacol Ther 88:223–230
Karonen T, Neuvonen PJ, Backman JT (2011) The CYP2C8 inhibitor gemfibrozil does not affect the pharmacokinetics of zafirlukast. Eur J Clin Pharmacol 67:151–155
Karonen T, Laitila J, Niemi M, Neuvonen PJ, Backman JT (2012) Fluconazole but not the CYP3A4 inhibitor, itraconazole, increases zafirlukast plasma concentrations. Eur J Clin Pharmacol 68:681–688
Kassahun K, Skordos K, McIntosh I, Slaughter D, Doss GA, Baillie TA, Yost GS (2005) Zafirlukast metabolism by cytochrome P450 3A4 produces an electrophilic alpha, beta-unsaturated iminium species that results in the selective mechanism-based inactivation of the enzyme. Chem Res Toxicol 18:1427–1437
Kelloway JS (1997) Zafirlukast: the first leukotriene-receptor antagonist approved for the treatment of asthma. Ann Pharmacother 31:1012–1021
Kirchheiner J, Störmer E, Meisel C, Steinbach N, Roots I, Brockmöller J (2003a) Influence of CYP2C9 genetic polymorphisms on pharmacokinetics of celecoxib and its metabolites. Pharmacogenetics 13:473–480
Kirchheiner J, Kudlicz D, Meisel C, Bauer S, Meineke I, Roots I, Brockmöller J (2003b) Influence of CYP2C9 polymorphisms on the pharmacokinetics and cholesterol-lowering activity of (−)−3S,5R-fluvastatin and (+)−3R,5S-fluvastatin in healthy volunteers. Clin Pharmacol Ther 74:186–194
Lee CR, Goldstein JA, Pieper JA (2002) Cytochrome P450 2C9 polymorphisms: a comprehensive review of the in vitro and human data. Pharmacogenetics 12:251–263
Lee HI, Bae JW, Choi CI, Lee YJ, Byeon JY, Jang CG, Lee SY (2014) Strongly increased exposure of meloxicam in CYP2C9*3/*3 individuals. Pharmacogenet Genomics 24:113–117
Lee YJ, Byeon JY, Kim YH, Kim SH, Choi CI, Bae JW, Sohn UD, Jang CG, Lee J, Lee SY (2015) Effects of CYP2C9*1/*3 genotype on the pharmacokinetics of flurbiprofen in Korean subjects. Arch Pharm Res 38:1232–1237
Li Z, Wang G, Wang LS, Zhang W, Tan ZR, Fan L, Chen BL, Li Q, Liu J, Tu JH, Hu DL, Liu ZQ, Zhou HH (2009) Effects of the CYP2C9*13 allele on the pharmacokinetics of losartan in healthy male subjects. Xenobiotica 39:788–793
Lipworth BJ (1999) Leucotriene-receptor antagonists. Lancet 353:57–62
Lundblad MS, Ohlsson S, Johansson P, Lafolie P, Eliasson E (2006) Accumulation of celecoxib with a 7-fold higher drug exposure in individuals homozygous for CYP2C9*3. Clin Pharmacol Ther 79:287–288
Maekawa K, Harakawa N, Sugiyama E, Tohkin M, Kim SR, Kaniwa N, Katori N, Hasegawa R, Yasuda K, Kamide K, Miyata T, Saito Y, Sawada J (2009) Substrate-dependent functional alterations of seven CYP2C9 variants found in Japanese subjects. Drug Metab Dispos 37:1895–1903
Michelson D, Read HA, Ruff DD, Witcher J, Zhang S, McCracken J (2007) CYP2D6 and clinical response to atomoxetine in children and adolescents with ADHD. J Am Acad Child Adolesc Psychiatry 46:242–251
Miners JO, Birkett DJ (1998) Cytochrome P4502C9: an enzyme of major importance in human drug metabolism. Br J Clin Pharmacol 45:525–538
Nakai K, Habano W, Nakai K, Fukushima N, Suwabe A, Moriya S, Osano K, Gurwitz D (2005) Ethnic differences in CYP2C9*2 (Arg144Cys) and CYP2C9*3 (Ile359Leu) genotypes in Japanese and Israeli populations. Life Sci 78:107–111
Savidge RD, Bui KH, Birmingham BK, Morse JL, Spreen RC (1998) Metabolism and excretion of zafirlukast in dogs, rats and mice. Drug Metab Dispos 26:1069–1076
Scott SA, Khasawneh R, Peter I, Kornreich R, Desnick RJ (2011) Combined CYP2C9, VKORC1 and CYP4F2 frequencies among racial and ethnic groups. Pharmacogenomics 11:781–791
Si D, Guo Y, Zhang Y, Yang L, Zhou H, Zhong D (2004) Identification of a novel variant CYP2C9 allele in Chinese. Pharmacogenetics 14:465–469
Stempak D, Bukaveckas BL, Linder M, Koren G, Baruchel S (2005) Cytochrome P450 2C9 genotype: impact on celecoxib safety and pharmacokinetics in a pediatric patient. Clin Pharmacol Ther 78:309–310
ter Laak MA, Temmink AH, Koeken A, van ‘t Veer NE, van Hattum PR, Cobbaert CM (2010) Recognition of impaired atomoxetine metabolism because of low CYP2D6 activity. Pediatr Neurol 43:159–162
Van Boonen D, Marsh S, McLeod H, Carrillo MW, Sangkuhl K, Klein TE, Altman RB (2010) Cytochrome P450 2C9-CYP2C9. Pharmacogenet Genomics 20:277–281
Vormfelde SV, Engelhardt S, Zirk A, Meineke I, Tuchen F, Kirchheiner J, Brockmöller J (2004) CYP2C9 polymorphisms and the interindividual variability in pharmacokinetics and pharmacodynamics of the loop diuretic drug torsemide. Clin Pharmacol Ther 76:557–566
Zhou YH, Zheng QC, Li ZS, Zhang Y, Sun M, Sun CC, Si D, Cai L, Guo Y, Zhou H (2006) On the human CYP2C9*13 variant activity reduction: a molecular dynamics simulation and docking study. Biochimie 88:1457–1465
Acknowledgments
This work was supported by the NRF grant funded by the Korea government (MSIP) (No. 2016R1A2B4007381).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no potential conflict of interest with respect to the authorship and/or publication of this article.
Additional information
Hyun-Jee Lee, Young-Hoon Kim, and Se-Hyung Kim, have contributed equally to this study.
Rights and permissions
About this article
Cite this article
Lee, HJ., Kim, YH., Kim, SH. et al. Effects of CYP2C9 genetic polymorphisms on the pharmacokinetics of zafirlukast. Arch. Pharm. Res. 39, 1013–1019 (2016). https://doi.org/10.1007/s12272-016-0785-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-016-0785-x