Abstract
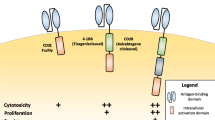
Chimeric antigen receptor-modified T cells (CAR-T) have emerged as a new modality for cancer immunotherapy due to their potent efficacy against terminal cancers. CAR-Ts are reported to exert higher efficacy than monoclonal antibodies and antibody–drug conjugates, and act via mechanisms distinct from T cell receptor-engineered T cells. These cells are constructed by transducing genes encoding fusion proteins of cancer antigen-recognizing single-chain Fv linked to intracellular signaling domains of T cell receptors. CAR-Ts are classified as first-, second- and third-generation, depending on the intracellular signaling domain number of T cell receptors. This review covers the current status of CAR-T research, including basic proof-of-concept investigations at the cell and animal levels. Currently ongoing clinical trials of CAR-T worldwide are additionally discussed. Owing to the lack of existing approved products, several unresolved concerns remain with regard to safety, efficacy and manufacturing of CAR-T, as well as quality control issues. In particular, the cytokine release syndrome is the major side-effect impeding the successful development of CAR-T in clinical trials. Here, we have addressed the challenges and regulatory perspectives of CAR-T therapy.







Similar content being viewed by others
References
Barrett DM, Liu X, Jiang S, June CH, Grupp SA, Zhao Y (2013) Regimen-specific effects of RNA-modified chimeric antigen receptor T cells in mice with advanced leukemia. Hum Gene Ther 24:717–727
Blat D, Zigmond E, Alteber Z, Waks T, Eshhar Z (2014) Suppression of murine colitis and its associated cancer by carcinoembryonic antigen-specific regulatory T cells. Mol Ther 22:1018–1028
Budde LE, Berger C, Lin Y, Wang J, Lin X, Frayo SE, Brouns SA, Spencer DM, Till BG, Jensen MC, Riddell SR, Press OW (2013) Combining a CD20 chimeric antigen receptor and an inducible caspase 9 suicide switch to improve the efficacy and safety of T cell adoptive immunotherapy for lymphoma. PLoS One 8:e82742
Casucci M, Bondanza A (2011) Suicide gene therapy to increase the safety of chimeric antigen receptor-redirected T lymphocytes. J Cancer 2:378–382
Chow KK, Naik S, Kakarla S, Brawley VS, Shaffer DR, Yi Z, Rainusso N, Wu MF, Liu H, Kew Y, Grossman RG, Powell S, Lee D, Ahmed N, Gottschalk S (2013) T cells redirected to EphA2 for the immunotherapy of glioblastoma. Mol Ther 21:629–637
Chu J, Deng Y, Benson DM, He S, Hughes T, Zhang J, Peng Y, Mao H, Yi L, Ghoshal K, He X, Devine SM, Zhang X, Caligiuri MA, Hofmeister CC, Yu J (2014) CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia 28:917–927
Davies DM, Foster J, Van Der Stegen SJ, Parente-Pereira AC, Chiapero-Stanke L, Delinassios GJ, Burbridge SE, Kao V, Liu Z, Bosshard-Carter L, Van Schalkwyk MC, Box C, Eccles SA, Mather SJ, Wilkie S, Maher J (2012) Flexible targeting of ErbB dimers that drive tumorigenesis by using genetically engineered T cells. Mol Med 18:565–576
Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska M, Qu J, Wasielewska T, He Q, Fink M, Shinglot H, Youssif M, Satter M, Wang Y, Hosey J, Quintanilla H, Halton E, Bernal Y, Bouhassira DC, Arcila ME, Gonen M, Roboz GJ, Maslak P, Douer D, Frattini MG, Giralt S, Sadelain M, Brentjens R (2014) Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 6:224ra25
Fransson M, Piras E, Burman J, Nilsson B, Essand M, Lu B, Harris RA, Magnusson PU, Brittebo E, Loskog AS (2012) CAR/FoxP3-engineered T regulatory cells target the CNS and suppress EAE upon intranasal delivery. J Neuroinflam 9:112
Gargett T, Brown MP (2014) The inducible caspase-9 suicide gene system as a “safety switch” to limit on-target, off-tumor toxicities of chimeric antigen receptor T cells. Front Pharmacol 5:235
Geldres C, Savoldo B, Hoyos V, Caruana I, Zhang M, Yvon E, Del Vecchio M, Creighton CJ, Ittmann M, Ferrone S, Dotti G (2014) T lymphocytes redirected against the chondroitin sulfate proteoglycan-4 control the growth of multiple solid tumors both in vitro and in vivo. Clin Cancer Res 20:962–971
Gill S, June CH (2015) Going viral: chimeric antigen receptor T-cell therapy for hematological malignancies. Immunol Rev 263:68–89
Grada Z, Hegde M, Byrd T, Shaffer DR, Ghazi A, Brawley VS, Corder A, Schönfeld K, Koch J, Dotti G, Heslop HE, Gottschalk S, Wels WS, Baker ML, Ahmed N (2013) TanCAR: a novel bispecific chimeric antigen receptor for cancer immunotherapy. Mol Ther Nucl Acids 2:e105
Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, Milone MC, Levine BL, June CH (2013) Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 368:1509–1518
Heczey A, Liu D, Tian G, Courtney AN, Wei J, Marinova E, Gao X, Guo L, Yvon E, Hicks J, Liu H, Dotti G, Metelitsa LS (2014) Invariant NKT cells with chimeric antigen receptor provide a novel platform for safe and effective cancer immunotherapy. Blood 124:2824–2833
Hu WX, Chen HP, Yu K, Shen LX, Wang SuSZ, Sui WJ, Shan DM, Li HZ (2012) Gene therapy of malignant solid tumors by targeting erbB2 receptors and by activating T cells. Cancer Biother Radiopharm 27:711–718
Huang G, Yu L, Cooper LJ, Hollomon M, Huls H, Kleinerman ES (2012) Genetically modified T cells targeting interleukin-11 receptor α-chain kill human osteosarcoma cells and induce the regression of established osteosarcoma lung metastases. Cancer Res 72:271–281
Hudecek M, Lupo-Stanghellini MT, Kosasih PL, Sommermeyer D, Jensen MC, Rader C, Riddell SR (2013) Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clin Cancer Res 19:3153–3164
Inaguma Y, Akahori Y, Murayama Y, Shiraishi K, Tsuzuki-Iba S, Endoh A, Tsujikawa Demachi-Okamura A, Hiramatsu K, Saji H, Yamamoto Y, Yamamoto N, Nishimura Y, Takahashi T, Kuzushima K, Emi N, Akatsuka Y (2014) Construction and molecular characterization of a T-cell receptor-like antibody and CAR-T cells specific for minor histocompatibility antigen HA-1H. Gene Ther 21:575–584
Jiang H, Zhang W, Shang P, Zhang H, Fu W, Ye F, Zeng T, Huang H, Zhang X, Sun W, Man-Yuen Sze D, Yi Q, Hou J (2014) Transfection of chimeric anti-CD138 gene enhances natural killer cell activation and killing of multiple myeloma cells. Mole Oncol 8:297–310
John LB, Kershaw MH, Darcy PK (2013) Blockade of PD-1 immunosuppression boosts CAR T-cell therapy. Oncoimmunol 2:e26286
Kakarla S, Gottschalk S (2014) CAR T cells for solid tumors: armed and ready to go? Cancer J 20:151–155
Kershaw MH, Westwood JA, Darcy PK (2013) Gene-engineered T cells for cancer therapy. Nat Rev Cancer 13:525–541
Kershaw MH, Westwood JA, Slaney CY, Darcy PK (2014) Clinical application of genetically modified T cells in cancer therapy. Clin Transl Immunol 3(5):e16
Kobayashi E, Kishi Ozawa T, Hamana H, Nakagawa H, Jin A, Lin Z, Muraguchi A (2014) A chimeric antigen receptor for TRAIL-receptor 1 induces apoptosis in various types of tumor cells. Biochem Biophys Res Com 453:798–803
Kong S, Sengupta S, Tyler B, Bais AJ, Ma Q, Doucette S, Zhou J, Sahin A, Carter BS, Brem H, Junghans RP, Sampath P (2012) Suppression of human glioma xenografts with second-generation IL13R-specific chimeric antigen receptor-modified T cells. Clin Cancer Res 18:5949–5960
Krebs S, Chow KK, Yi Z, Rodriguez-Cruz T, Hegde M, Gerken C, Ahmed N, Gottschalk S (2014) T cells redirected to interleukin-13Rα2 with interleukin-13 mutein-chimeric antigen receptors have anti-glioma activity but also recognize interleukin-13Rα1. Cytothera 16:1121–1131
Kumaresan PR, Manuri PR, Albert ND, Maiti S, Singh H, Mi T, Roszik J, Rabinovich B, Olivares S, Krishnamurthy J, Zhang L, Najjar AM, Huls MH, Lee DA, Champlin RE, Kontoyiannis DP, Cooper LJ (2014) Bioengineering T cells to target carbohydrate to treat opportunistic fungal infection. Proc Natl Acad Sci USA 111:10660–10665
Lanitis E, Poussin M, Hagemann IS, Coukos G, Sandaltzopoulos R, Scholler N, Powell DJ (2012) Redirected antitumor activity of primary human lymphocytes transduced with a fully human anti-mesothelin chimeric receptor. Mol Ther 20:633–643
Lanitis E, Poussin M, Klattenhoff AW, Song D, Sandaltzopoulos R, June CH, Powell DJ (2013) Chimeric antigen receptor T Cells with dissociated signaling domains exhibit focused antitumor activity with reduced potential for toxicity in vivo. Cancer Immunol Res 1:43–53
Lee DW, Barrett DM, Mackall C, Orentas R, Grupp SA (2012) The future is now: chimeric antigen receptors as new targeted therapies for childhood cancer. Clin Cancer Res 18:2780–2790
Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M, Shah NN, Steinberg SM, Stroncek D, Tschernia N, Yuan C, Zhang H, Zhang L, Rosenberg SA, Wayne AS, Mackall CL (2015) T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 385:517–528
Levine BL (2015) Performance-enhancing drugs: design and production of redirected chimeric antigen receptor (CAR) T cells. Cancer Gene Ther 22:79–84
Ma Q, Safar M, Holmes E, Wang Y, Boynton AL, Junghans RP (2014) Anti-prostate specific membrane antigen designer T cells for prostate cancer therapy. Prostate 61:12–25
Maher J (2014) Clinical immunotherapy of B-cell malignancy using CD19-targeted CAR T-cells. Curr Gene Ther 14:35–43
Mata M, Vera JF, Gerken C, Rooney CM, Miller T, Pfent C, Wang LL, Wilson-Robles HM, Gottschalk S (2014) Toward immunotherapy with redirected T cells in a large animal model: ex vivo activation, expansion, and genetic modification of canine T cells. J Immunother 37:407–415
Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD, Melenhorst JJ, Rheingold SR, Shen A, Teachey DT, Levine BL, June CH, Porter DL, Grupp SA (2014) Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371:1507–1517
Maude SL, Teachey DT, Porter DL, Grupp SA (2015) CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood 125:4017–4023
Maus MV, Grupp SA, Porter DL, June CH (2014) Antibody-modified T cells: CARs take the front seat for hematologic malignancies. Blood 123:2625–2635
Morgan RA, Johnson LA, Davis JL, Zheng Z, Woolard KD, Reap EA, Feldman SA, Chinnasamy N, Kuan CT, Song H, Zhang W, Fine HA, Rosenberg SA (2012) Recognition of glioma stem cells by genetically modified T cells targeting EGFRvIII and development of adoptive cell therapy for glioma. Human Gene Ther 23:1043–1053
Nishio N, Diaconu I, Liu H, Cerullo V, Caruana I, Hoyos V, Bouchier-Hayes L, Savoldo B, Dotti G (2014) Armed oncolytic virus enhances immune functions of chimeric antigen receptor-modified T cells in solid tumors. Cancer Res 74:5195–5205
Puri RK (2014) FDA Perspective on the regulation of TCR/CAR T-cell products. In: 12th CIMT (Cancer Immunotherapy) annual symposium
Rainusso N, Brawley VS, Ghazi A, Hicks MJ, Gottschalk S, Rosen JM, Ahmed N (2012) Immunotherapy targeting HER2 with genetically modified T cells eliminates tumor-initiating cells in osteosarcoma. Cancer Gene Ther 19:212–217
Ritchie DS, Neeson PJ, Khot A, Peinert S, Tai T, Tainton K, Chen K, Shin M, Wall DM, Hönemann D, Gambell P, Westerman DA, Haurat J, Westwood JA, Scott AM, Kravets L, Dickinson M, Trapani JA, Smyth MJ, Darcy PK, Kershaw MH, Prince HM (2013) Persistence and efficacy of second generation CAR T cell against the LeY antigen in acute myeloid leukemia. Mol Ther 21:2122–2129.HM
Saito S, Nakazawa Y, Sueki A, Matsuda K, Tanaka M, Yanagisawa R, Maeda Y, Sato Y, Okabe S, Inukai T, Sugita K, Wilson MH, Rooney CM, Koike K (2014) Anti-leukemic potency of piggyBac-mediated CD19-specific T cells against refractory Philadelphia chromosome-positive acute lymphoblastic leukemia. Cytother 6:1257–1269
Schölzel S, Zimmermann W, Schwarzkopf G, Grunert F, Rogaczewski B, Thompson J (2000) Carcinoembryonic antigen family members CEACAM6 and CEACAM7 are differentially expressed in normal tissues and oppositely deregulated in hyperplastic colorectal polyps and early adenomas. Am J Pathol 156:595–605
Schuberth PC, Jakka G, Jensen SM, Wadle A, Gautschi F, Haley D, Haile S, Mischo A, Held G, Thiel M, Tinguely M, Bifulco CB, Fox BA, Renner C, Petrausch U (2013) Effector memory and central memory NY-ESO-1-specific re-directed T cells for treatment of multiple myeloma. Gene Ther 20:386–395
Shin JH, Park HB, Oh YM, Lim DP, Lee JE, Seo HH, Lee SJ, Eom HS, Kim IH, Lee SH, Choi K (2012) Positive conversion of negative signaling of CTLA4 potentiates antitumor efficacy of adoptive T-cell therapy in murine tumor models. Blood 119:5678–5687
Sun M, Shi H, Liu C, Liu J, Liu X, Sun Y (2014) Construction and evaluation of a novel humanized HER2-specific chimeric receptor. Breast Cancer Res 16:R61
Tamada K, Geng D, Sakoda Y, Bansal N, Srivastava R, Li Z, Davila E (2012) Redirecting gene-modified T cells toward various cancer types using tagged antibodies. Clin Cancer Res 18:6436–6445
Torikai H, Reik A, Liu PQ, Zhou Y, Zhang L, Maiti S, Huls H, Miller JC, Kebriaei P, Rabinovitch B, Lee DA, Champlin RE, Bonini C, Naldini L, Rebar EJ, Gregory PD, Holmes MC, Cooper LJ (2012) A foundation for universal T-cell based immunotherapy: T cells engineered to express a CD19-specific chimeric-antigen-receptor and eliminate expression of endogenous TCR. Blood 119:5697–5705
Tsukahara TN, Iwase N, Kawakami K, Iwasaki M, Yamamoto C, Ohmine K, Uchibori R, Teruya T, Ido H, Saga Y, Urabe M, Mizukami H, Kume A, Nakamura M, Brentjens R, Ozawa K (2015) The Tol2 transposon system mediates the genetic engineering of T-cells with CD19-specific chimeric antigen receptors for B-cell malignancies. Gene Ther 22:209–215
Wang LX, Westwood JA, Moeller M, Duong CP, Wei WZ, Malaterre J, Trapani JA, Neeson P, Smyth MJ, Kershaw MH, Darcy PK (2010) Tumor ablation by gene-modified T cells in the absence of autoimmunity. Cancer Res 70:9591–9598
Wang X, Rivière I (2015) Manufacture of tumor- and virus-specific T lymphocytes for adoptive cell therapies. Cancer Gene Ther 22:85–94
Wang X, Naranjo A, Brown CE, Bautista C, Wong CW, Chang WC, Aguilar B, Ostberg JR, Riddell SR, Forman SJ, Jensen MC (2012) Phenotypic and functional attributes of lentivirus-modified CD19-specific human CD8 + central memory T cells manufactured at clinical scale. J Immunother 35:689–701
Wang C, Hu W, Shen L, Dou R, Zhao S, Shan D, Yu K, Huang R, Li H (2014a) Adoptive antitumor immunotherapy in vitro and in vivo using genetically activated erbB2-specific T cells. J Immunother 37:351–359
Wang Y, Zhang WY, Han QW, Liu Y, Dai HR, Guo YL, Bo J, Fan H, Zhang Y, Zhang YJ, Chen MX, Feng KC, Wang QS, Fu XB, Han WD (2014b) Effective response and delayed toxicities of refractory advanced diffuse large B-cell lymphoma treated by CD20-directed chimeric antigen receptor-modified T cells. Clin Immunol 155:160–175
Watanabe K, Terakura S, Martens AC, van Meerten T, Uchiyama S, Imai M, Sakemura R, Goto T, Hanajiri R, Imahashi N, Shimada K, Tomita A, Kiyoi H, Nishida T, Naoe T, Murata M (2015) Target antigen density governs the efficacy of anti-CD20-CD28-CD3 ζ chimeric antigen receptor-modified effector CD8+ T cells. J Immunol 94:911–920
Xu Y, Zhang M, Ramos CA, Durett A, Liu E, Dakhova O, Liu H, Creighton CJ, Gee AP, Heslop HE, Rooney CM, Savoldo B, Dotti G (2014) Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15. Blood 123:3750–3759
Zhao YE, Moon E, Carpenito C, Paulos CM, Liu X, Brennan AL, Chew A, Carroll RG, Scholler J, Levine BL, Albelda SM, June CH (2010) Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res 70:9053–9061
Acknowledgments
This research was supported by a grant (15172MFDS163) from Ministry of Food and Drug Safety in 2015.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, MG., Kim, D., Suh, SK. et al. Current status and regulatory perspective of chimeric antigen receptor-modified T cell therapeutics. Arch. Pharm. Res. 39, 437–452 (2016). https://doi.org/10.1007/s12272-016-0719-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-016-0719-7