Abstract
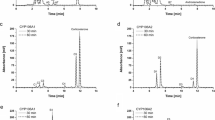
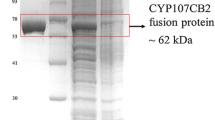
In this study, we examined the catalytic activity of CYP106A1 from the Bacillus megaterium American Type Culture Collection 14581 strain. The CYP106A1 gene was cloned from B. megaterium, heterologously expressed in Escherichia coli, and purified. Potential electron partners and possible bacterial CYP106A1 substrates were identified by examining the oxidative activity toward a set of steroids in the presence of several reductase systems. The activities of CYP106A1 in a reconstituted system could not be achieved using rat NADPH-P450 reductase or a putidaredoxin reductase–putidaredoxin pair. However, the spinach redox proteins, a ferredoxin reductase–ferredoxin pair, were found to be efficient redox partners for CYP106A1. CYP106A1 catalyzes the hydroxylation of a set of steroids including testosterone, progesterone, 17α-hydroxyprogesterone, 11-deoxycorticosterone, corticosterone, and 11-deoxycortisol to produce monohydroxylated products as the major metabolites. These results suggest that CYP106A1 would be useful for the bioconversion of steroid hormones to hydroxylated products that can be used for industrial applications.







Similar content being viewed by others
References
Berg, A., J.A. Gustafsson, and M. Ingelman-Sundberg. 1976. Characterization of a cytochrome P-450-dependent steroid hydroxylase system present in Bacillus megaterium. Journal of Biological Chemistry 251: 2831–2838.
Bernhardt, R. 2006. Cytochromes P450 as versatile biocatalysts. Journal of Biotechnology 124: 128–145.
Bleif, S., F. Hannemann, J. Zapp, D. Hartmann, J. Jauch, and R. Bernhardt. 2012. A new Bacillus megaterium whole-cell catalyst for the hydroxylation of the pentacyclic triterpene 11-keto-β-boswellic acid (KBA) based on a recombinant cytochrome P450 system. Applied Microbiology and Biotechnology 93: 1135–1146.
Brill, E., F. Hannemann, J. Zapp, G. Brüning, J. Jauch, and R. Bernhardt. 2013. A new cytochrome P450 system from Bacillus megaterium DSM319 for the hydroxylation of 11-keto-β-boswellic acid (KBA). Applied Microbiology and Biotechnology. doi:10.1007/s00253-013-5029-0.
Farinas, E.T., U. Schwaneberg, A. Glieder, and F.H. Arnold. 2001. Directed evolution of a cytochrome P450 monooxygenase for alkane oxidation. Advanced Synthesis & Catalysis 343: 601–606.
Fernandes, P., A. Cruz, B. Angelova, H.M. Pinheiro, and J.M.S. Cabral. 2003. Microbial conversion of steroid compounds: Recent developments. Enzyme and Microbial Technology 32: 688–705.
Goñi, G., A. Zöllner, M. Lisurek, A. Velázquez-Campoy, S. Pinto, C. Gómez-Moreno, F. Hannemann, R. Bernhardt, and M. Medina. 2009. Cyanobacterial electron carrier proteins as electron donors to CYP106A2 from Bacillus megateriumATCC 13368. Biochimica et Biophysica Acta 1794: 1635–1642.
Guengerich, F.P. 1991. Reactions and significance of cytochrome P-450 enzymes. Journal of Biological Chemistry 266: 10019–10022.
Guengerich, F.P. 2001. Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity. Chemical Research in Toxicology 14: 611–650.
Guengerich, F.P., and A.W. Munro. 2013. Unusual cytochrome p450 enzymes and reactions. Journal of Biological Chemistry 288: 17065–17073.
He, J.S., R.T. Ruettinger, H.M. Liu, and A.J. Fulco. 1989. Molecular cloning, coding nucleotides and the deduced amino acid sequence of P-450BM-1 from Bacillus megaterium. Biochimica et Biophysica Acta 1009: 301–303.
Holland, H.L. 1999. Recent advances in applied and mechanistic aspects of the enzymatic hydroxylation of steroids by whole-cell biocatalysts. Steroids 64: 178–186.
Hosea, N.A., G.P. Miller, and F.P. Guengerich. 2000. Elucidation of distinct ligand binding sites for cytochrome P450 3A4. Biochemistry 39: 5929–5939.
Joo, H., Z. Lin, and F.H. Arnold. 1999. Laboratory evolution of peroxide-mediated cytochrome P450 hydroxylation. Nature 399: 670–673.
Kang, J.Y., S.Y. Kim, D. Kim, D.H. Kim, S.M. Shin, S.H. Park, K.H. Kim, H.C. Jung, J.G. Pan, Y.H. Joung, Y.T. Chi, H.Z. Chae, T. Ahn, and C.H. Yun. 2011. Characterization of diverse natural variants of CYP102A1 found within a species of Bacillus megaterium. AMB Express 1: 1–12.
Kelly, S.L., D.E. Kelly, C.J. Jackson, A.G.S. Warrilow, and D.C. Lamb. 2005. The diversity and importance of microbial cytochromes P450. In Cytochrome P450: Structure, mechanism, and biochemistry, 3rd ed, ed. P.R. Ortiz de Montellano, 585–617. New York: Kluwer Academic/Plenum Publishers.
Kim, D., and P.R. Ortiz de Montellano. 2009. Tricistronic overexpression of cytochrome P450cam, putidaredoxin, and putidaredoxin reductase provides a useful cell-based catalytic system. Biotechnology Letters 31: 1427–1431.
Kim, D.H., K.H. Kim, E.M. Isin, F.P. Guengerich, H.Z. Chae, T. Ahn, and C.H. Yun. 2008. Heterologous expression and characterization of wild-type human cytochrome P450 1A2 without conventional N-terminal modification in Escherichia coli. Protein Expression and Purification 57: 188–200.
Lisurek, M., M.J. Kang, R.W. Hartmann, and R. Bernhardt. 2004. Identification of monohydroxyprogesterones produced by CYP106A2 using comparative HPLC and electrospray ionisation collision-induced dissociation mass spectrometry. Biochemical and Biophysical Research Communications 319: 677–682.
Mahato, S.B., and S. Gari. 1997. Advances in microbial steroid biotransformation. Steroids 62: 332–345.
Omura, T., and R. Sato. 1964. The carbon monoxide-binding pigment of liver microsomes. II. Solubilization, purification, and properties. Journal of Biological Chemistry 239: 2379–2385.
Rauschenbach, R., M. Isernhagen, C. Noeske-Jungblut, W. Boidol, and G. Siewert. 1993. Cloning sequencing and expression of the gene for cytochrome P450 meg, the steroid-15 beta-monooxygenase from Bacillus megaterium ATCC 13368. Molecular and General Genetics 241: 170–176.
Sedlaczek, L. 1988. Biotransformation of steroids. Critical Reviews in Biotechnology 7: 187–236.
Shen, A.L., T.D. Porter, T.E. Wilson, and C.B. Kasper. 1989. Structural analysis of the FMN binding domain of NADPH-cytochrome P-450 oxidoreductase by site-directed mutagenesis. Journal of Biological Chemistry 264: 7584–7589.
Urlacher, V.B., and S. Eiben. 2006. Cytochrome P450 monooxygenases: perspectives for synthetic application. Trends in Biotechnology 24: 324–330.
Virus, C., and R. Bernhardt. 2008. Molecular evolution of a steroid hydroxylating cytochrome P450 using a versatile steroid detection system for screening. Lipids 43: 1133–1141.
Virus, C., M. Lisurek, B. Simgen, F. Hannemann, and R. Bernhardt. 2006. Function and engineering of the 15beta-hydroxylase CYP106A2. Biochemical Society Transactions 34: 1215–1218.
Yun, C.H., G.P. Miller, and F.P. Guengerich. 2000. Rate-determining steps in phenacetin oxidations by human cytochrome P450 1A2 and selected mutants. Biochemistry 39: 11319–11329.
Zehentgruber, D., F. Hannemann, S. Bleif, R. Bernhardt, and S. Lütz. 2010. Towards preparative scale steroid hydroxylation with cytochrome P450 monooxygenase CYP106A2. ChemBioChem 11: 713–721.
Acknowledgments
This research was supported by the Next-Generation BioGreen 21 program (SSAC, Grant #: PJ00948302), Rural Development Administration, Republic of Korea; and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2013R1A1A2007090), Republic of Korea.
Conflict of interest
The authors declare no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, GY., Kim, DH., Kim, D. et al. Functional characterization of steroid hydroxylase CYP106A1 derived from Bacillus megaterium . Arch. Pharm. Res. 38, 98–107 (2015). https://doi.org/10.1007/s12272-014-0366-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-014-0366-9