Abstract
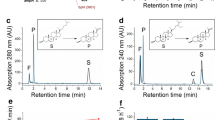
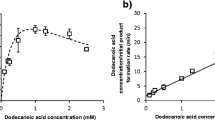
The use of cytochromes P450 for the regio- and stereoselective hydroxylation of non-activated carbon atoms in biotechnological applications reflects an efficient and cost-effective alternative in comparison to classical organic chemistry. The prokaryotic cytochrome P450 CYP106A2 from Bacillus megaterium ATCC 13368 hydroxylates a variety of 3-oxo-Δ4 steroids and recently it was identified to carry out a one-step regioselective allylic hydroxylation of the diterpene abietic acid. The anti-inflammatory pentacyclic triterpene 11-Keto-β-boswellic acid (KBA) was found to be a further substrate of CYP106A2, being the first report of a pentacyclic triterpene conversion by a prokaryotic P450. The reaction products were analyzed by HPLC and the corresponding kinetic parameters were investigated. Structure determination of the main product by NMR revealed a 15α-hydroxylation of this substrate. In order to overcome the inability of a recombinant P450 whole-cell system in E. coli for the uptake of acids with terpene structure, we developed for the first time an expression system for cytochromes P450 in B. megaterium (strains MS941 and ATCC 13368). Interestingly, CYP106A2 was only successfully expressed in the plasmid-less B. megaterium strain MS941 but not in ATCC13368. This recombinant system, with the co-expressed heterologous redox chain of the P450, bovine adrenodoxin reductase (AdR), and bovine adrenodoxin (Adx), was applied for the whole-cell conversion of KBA. The formation of 15α-hydroxy-KBA was increased 15-fold in comparison with the naturally CYP106A2-expressing B. megaterium strain ATCC 13368.








Similar content being viewed by others

References
Barg H, Malten M, Jahn M, Jahn D (eds) (2005) Protein and vitamin production in Bacillus megaterium vol 18. Microbial processes and products, 1st edn. Humana Press, Inc, Totowa
Berg A, Carlstrom K, Gustafsson JA, Ingelman-Sundberg M (1975) Demonstration of a cytochrome P-450-dependent steroid 15beta-hydroxylase in Bacillus megaterium. Biochem Biophys Res Commun 66(4):1414–1423
Berg A, Gustafsson JA, Ingelman-Sundberg M (1976) Characterization of a cytochrome P-450-dependent steroid hydroxylase system present in Bacillus megaterium. J Biol Chem 251(9):2831–2838
Berg A, Ingelman-Sundberg M, Gustafsson JA (1979) Isolation and characterization of cytochrome P-450meg. Acta Biol Med Ger 38(2–3):333–344
Bernhardt R (1996) Cytochrome P450: structure, function, and generation of reactive oxygen species. Rev Physiol Biochem Pharmacol 127:137–221
Bernhardt R (2006) Cytochromes P450 as versatile biocatalysts. J Biotechnol 124(1):128–145
Bleif S, Hannemann F, Lisurek M, von Kries JP, Zapp J, Dietzen M, Antes I, Bernhardt R (2011) Identification of CYP106A2 as a regioselective allylic bacterial diterpene hydroxylase. Chembiochem. doi:10.1002/cbic.201000404
Chefson A, Auclair K (2006) Progress towards the easier use of P450 enzymes. Mol Biosyst 2(10):462–469
Cirino PC, Arnold FH (2003) A self-sufficient peroxide-driven hydroxylation biocatalyst. Angew Chem Int Ed Engl 42(28):3299–3301
Coon MJ, Vaz AD, Bestervelt LL (1996) Cytochrome P450 2: peroxidative reactions of diversozymes. FASEB J 10(4):428–434
Gamer M, Frode D, Biedendieck R, Stammen S, Jahn D (2009) A T7 RNA polymerase-dependent gene expression system for Bacillus megaterium. Appl Microbiol Biotechnol 82(6):1195–1203
Goni G, Zollner A, Lisurek M, Velazquez-Campoy A, Pinto S, Gomez-Moreno C, Hannemann F, Bernhardt R, Medina M (2009) Cyanobacterial electron carrier proteins as electron donors to CYP106A2 from Bacillus megaterium ATCC 13368. Biochim Biophys Acta 1794(11):1635–1642
Grote A, Hiller K, Scheer M, Munch R, Nortemann B, Hempel DC, Jahn D (2005) JCat: a novel tool to adapt codon usage of a target gene to its potential expression host. Nucleic Acids Res 33 (Web Server issue):W526-531
Gupta I, Parihar A, Malhotra P, Gupta S, Ludtke R, Safayhi H, Ammon HP (2001) Effects of gum resin of Boswellia serrata in patients with chronic colitis. Planta Med 67(5):391–395
Hannemann F, Virus C, Bernhardt R (2006) Design of an Escherichia coli system for whole cell mediated steroid synthesis and molecular evolution of steroid hydroxylases. J Biotechnol 25:25
Hollmann F, Withold B, Schmid A (2002) [cp*Rh(bpy)(H2O)]2+: a versatile tool for efficient and non-enzymatic regeneration of nicotinamide and flavin coenzymes. J Mol Cat B Enzym 791:1–10
Jauch J, Bergmann J (2003) An efficient method for large-scale preparation of 3-O-Acetyl-11-oxo-β-boswellic acid and other boswellic acids. Eur J Org Chem 2003:4752–4756
Jefcoate CR (1978) Measurement of substrate and inhibitor binding to microsomal cytochrome P-450 by optical-difference spectroscopy. Methods Enzymol 52:258–279
Krüger P, Daneshfar R, Eckert GP, Klein J, Volmer DA, Bahr U, Muller WE, Karas M, Schubert-Zsilavecz M, Abdel-Tawab M (2008) Metabolism of boswellic acids in vitro and in vivo. Drug Metab Dispos 36(6):1135–1142
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685
Lisurek M, Kang MJ, Hartmann RW, Bernhardt R (2004) Identification of monohydroxy progesterones produced by CYP106A2 using comparative HPLC and electrospray ionisation collision-induced dissociation mass spectrometry. Biochem Biophys Res Commun 319(2):677–682
Lisurek M, Simgen B, Antes I, Bernhardt R (2008) Theoretical and experimental evaluation of a CYP106A2 low homology model and production of mutants with changed activity and selectivity of hydroxylation. Chembiochem 9(9):1439–1449
Maurer SC, Kühnel K, Kaysser LA, Eiben S, Schmid RD, Urlacher VB (2005) Catalytic hydroxylation in biphasic systems using CYP102A1 mutants. Adv Synth Catal 347:1090–1098
Maurer SC, Schulze H, Schmid RD, Urlacher V (2003) Immobilisation of P450 BM-3 and an NADP+ cofactor recycling system: towards a technical application of heme-containing monooxygenaes in fine chemical synthesis. Adv Synth Catal 345:802–810
Poeckel D, Werz O (2006) Boswellic acids: biological actions and molecular targets. Curr Med Chem 13(28):3359–3369
Rauschenbach R, Isernhagen M, Noeske-Jungblut C, Boidol W, Siewert G (1993) Cloning sequencing and expression of the gene for cytochrome P450meg, the steroid-15 beta-monooxygenase from Bacillus megaterium ATCC 13368. Mol Gen Genet 241(1–2):170–176
Sagara Y, Wada A, Takata Y, Waterman MR, Sekimizu K, Horiuchi T (1993) Direct expression of adrenodoxin reductase in Escherichia coli and the functional characterization. Biol Pharm Bull 16(7):627–630
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, vol 3. CSHL, Cold Spring Harbor
Schenkman JB, Jansson I (1998) Spectral analyses of cytochromes P450. Methods Mol Biol 107:25–33
Simgen B, Contzen J, Schwarzer R, Bernhardt R, Jung C (2000) Substrate binding to 15beta-hydroxylase (CYP106A2) probed by FT infrared spectroscopic studies of the iron ligand CO stretch vibration. Biochem Biophys Res Commun 269(3):737–742
Stammen S, Muller BK, Korneli C, Biedendieck R, Gamer M, Franco-Lara E, Jahn D (2010) High-yield intra- and extracellular protein production using Bacillus megaterium. Appl Environ Microbiol 76(12):4037–4046
Syrovets T, Buchele B, Gedig E, Slupsky JR, Simmet T (2000) Acetyl-boswellic acids are novel catalytic inhibitors of human topoisomerases I and IIalpha. Mol Pharmacol 58(1):71–81
Szczebara FM, Chandelier C, Villeret C, Masurel A, Bourot S, Duport C, Blanchard S, Groisillier A, Testet E, Costaglioli P, Cauet G, Degryse E, Balbuena D, Winter J, Achstetter T, Spagnoli R, Pompon D, Dumas B (2003) Total biosynthesis of hydrocortisone from a simple carbon source in yeast. Nat Biotechnol 21(2):143–149
Uhlmann H, Beckert V, Schwarz D, Bernhardt R (1992) Expression of bovine adrenodoxin in E. coli and site-directed mutagenesis of /2 Fe-2S/ cluster ligands. Biochem Biophys Res Commun 188(3):1131–1138
Urlacher VB, Lutz-Wahl S, Schmid RD (2004) Microbial P450 enzymes in biotechnology. Appl Microbiol Biotechnol 64(3):317–325
Vary PS (1994) Prime time for Bacillus megaterium. Microbiology 140(Pt 5):1001–1013
Vary PS, Biedendieck R, Fuerch T, Meinhardt F, Rohde M, Deckwer WD, Jahn D (2007) Bacillus megaterium—from simple soil bacterium to industrial protein production host. Appl Microbiol Biotechnol 76(5):957–967
Virus C, Bernhardt R (2008) Molecular evolution of a steroid hydroxylating cytochrome P450 using a versatile steroid detection system for screening. Lipids 43(12):1133–41
Wittchen KD, Meinhardt F (1995) Inactivation of the major extracellular protease from Bacillus megaterium DSM319 by gene replacement. Appl Microbiol Biotechnol 42(6):871–877
Yang Y, Malten M, Grote A, Jahn D, Deckwer WD (2007) Codon optimized Thermobifida fusca hydrolase secreted by Bacillus megaterium. Biotechnol Bioeng 96(4):780–794
Zehentgruber D, Hannemann F, Bleif S, Bernhardt R, Lutz S (2010) Towards preparative scale steroid hydroxylation with cytochrome P450 monooxygenase CYP106A2. Chembiochem 11(5):713–721
Acknowledgment
The authors thank Prof. Dr. Dieter Jahn (TU Braunschweig) for the kind provision of the B. megaterium strain MS941 and the plasmid pKMBm4 and Dr. Simon Stammen (TU Braunschweig) for constructive discussions. We are thankful to Katharina Bompais and Wolfgang Reinle for the expression and excellent purification of CYP106A2, AdR, and Adx.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 68 kb)
Rights and permissions
About this article
Cite this article
Bleif, S., Hannemann, F., Zapp, J. et al. A new Bacillus megaterium whole-cell catalyst for the hydroxylation of the pentacyclic triterpene 11-keto-β-boswellic acid (KBA) based on a recombinant cytochrome P450 system. Appl Microbiol Biotechnol 93, 1135–1146 (2012). https://doi.org/10.1007/s00253-011-3467-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3467-0