Abstract
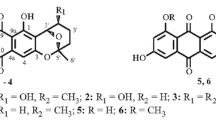
Two new anthraquinone dimers, melrubiellin A (1) and melrubiellin B (2), were isolated from the aerial part of Melandrium firmum Rohrbach, along with seven known compounds (3–9). The structures of these compounds were elucidated by spectral analyses, including 1D and 2D NMR (COSY, HMQC, HMBC and NOESY) experiments. Compound 1 and 2 exhibited significant cytotoxicity towards HeLa, NCI-H460, Hep G2, Hep 3B and MKN-28 cell lines with IC50 values ranging from 5.26 to 81.16 μM.


Similar content being viewed by others
References
Arnone, A., L. Camarda, G. Nasini, and G. Assante. 1986. Secondary mould metabolites. Part 15. Structure elucidation of rubellins A and B, two novel anthraquinone metabolites from Mycosphaerella rubella. Journal of the Chemical Society, Perkin Transactions 1: 255–260.
Carolina, E.M., M.L. Concepción, H.O. Simón, L.V. María, G. Dino, G.E. Raúl, and R. William. 2012. 1H and 13C NMR characterization of new cycloartane triterpenes from Mangifera indica. Magnetic Resonance in Chemistry 50: 52–57.
Chang, I.S., Y.B. Han, W.S. Woo, S.S. Kang, H. Lotter, and H. Wagner. 1989. Sapogenins from Melandrium firmum. Planta Medica 55: 544–547.
Chen, Y.F., P.C. Kuo, H.H. Chan, I.J. Kuo, F.W. Lin, C.R. Su, M.L. Yang, D.T. Li, and T.S. Wu. 2010. β-Carboline alkaloids from Stellaria dichotoma var. lanceolata and their anti-inflammatory activity. Journal of Natural Products 73: 1993–1998.
Cui, S.N. 1995. Chaoyaozhi, 77–79. Yanji: Yanbian People’s Press.
Darmograi, V.N. 1977. Flavonoids from certain species of the genus Melandrium. Khimiia Prirodnykh Soedinenii 1: 115–116.
Isaka, M., S. Palasarn, P. Tobwor, T. Boonruangprapa, and K. Tasanathai. 2012. Bioactive anthraquinone dimmers from the leafhopper pathogenic fungus Torrubiella sp. BBC28517. Journal of Antibiotics 65: 571–574.
Lee, M.Y., I.S. Shin, C.S. Seo, N.H. Lee, H.K. Ha, J.K. Son, and H.K. Shin. 2012. Effect of methanolic extract on testosterone-induced benign prostatic hypeiplasia in Wistar rats. Asian Journal of Andrology 14: 320–324.
Li, Q.C., H. Liang, Y.Y. Zhao, R.Y. Zhang, and L.H. Zhang. 1997. The chemical constituents from the roots of Bupleurum chinense. Journal of Chinese Pharmaceutical Sciences 6: 165–167.
Miethbauer, S., W. Günther, K.U. Schmidtke, I. Heiser, S. Gräfe, B. Gitter, and B. Liebermann. 2008. Uredinorubellins and caeruleoramularin, photodynamically active anthraquinone derivatives produced by two species of the genus Ramularia. Journal of Natural Products 71: 1371–1375.
Nemet, I., and L. Varga-Defterdarovic. 2007. Methylglyoxal-derived β-carbolines formed from tryptophan and its derivates in the maillard reaction. Amino Acids 32: 291–293.
Pei, Y.G., Q.X. Wu, and Y.P. Shi. 2007. Triterpenoids and other constituents from Euphorbia humifusa. Journal of the Chinese Chemical Society 54: 1565–1572.
Perry, L.M. 1980. Medicinal plants of east and southeast Asia, Attributed properties and uses, 74. Cambridge: MIT Press.
Sano, T., S. Takeuchi, T. Nakagawa, D. Ishikawa, S. Nanjo, T. Yamada, T. Nakamura, K. Matsumoto, and S. Yano. 2013. The novel phosphoinositide 3-kinase-mammalian target of rapamycin inhibitor, BEZ235, circumvents erlotinib resistance of epidermal growth factor receptor mutant lung cancer cells triggered by hepatocyte growth factor. International Journal of Cancer 133: 505–513.
Woo, E.H., and W.S. Woo. 1989. Flavonoid glycosides from Melandrium firmum. Archives of Pharmacal Research 12: 223–225.
Woo, E.H., and W.S. Woo. 1991. A novel prosapogenin from the methanolyzate of Melandrium crude saponins. Saengyak Hakhoechi 22: 211–214.
Woo, E.H., and W.S. Woo. 1992. Melandrioside A, a saponin from Melandrium firmum. Journal of Natural Products 55: 786–794.
Woo, W.S., J.S. Choi, and H.S. Chang. 1985. Phytochemical study on Melandrium firrnum. Archives of Pharmacal Research 8: 133–137.
Woo, W.S., and J.S. Choi. 1987. A phenolic amide and other constituents of Melandrium firmum. Phytochemistry 26: 2099–2100.
Zhang, L.J., X.D. Yang, L.Z. Xu, Z.M. Zou, and S.L. Yang. 2005. A new sterol glycoside from Securidaca inappendiculata. Journal of Asian Natural Products Research 7: 649–653.
Zhao, J.L., M. Yan, T.J. Shan, L. Yan, L.G. Zhou, M.G. Wang, and J.G. Wang. 2010. Antimicrobial metabolites from the endophytic fungus Pichia gulliermondii isolated from Paris polyphylla var. yunnanensis. Molecules 15: 7961–7970.
Zheng, M.S., N.K. Hwang, D.H. Kim, T.C. Moon, J.K. Son, and H.W. Chang. 2008. Chemical constituents of Melandrium firmum Rohrbach and their anti-inflammatory activity. Archives of Pharmacal Research 31: 318–322.
Acknowledgments
This work was supported by the Grant from the National Natural Science Foundation of China (No. 81160386, 81260474).
Author information
Authors and Affiliations
Corresponding author
Additional information
Chang-Hao Zhang and Da-Lei Yao have contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, CH., Yao, DL., Li, CS. et al. Cytotoxic anthraquinone dimers from Melandrium firmum . Arch. Pharm. Res. 38, 1033–1037 (2015). https://doi.org/10.1007/s12272-014-0360-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-014-0360-2