Abstract
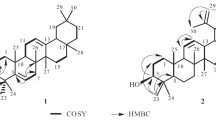
A bioassay-guided fractionation of the CH2Cl2 extract of Selaginella tamariscina yielded six sterols 1–6 such as (4α, 5α)-4, 14-dimethylcholest-8-en-3-one (1), ergosta-4, 6, 8(14), 22-tetraene-3-one (2), ergosterol endoperoxide (3), 7β-hydroxycholesterol (4), 7β-hydroxysitosterol (5), and 7α-hydroxysitosterol (6). The structures of isolated compounds were determined using spectroscopic methods. Among these isolates, compounds 2–5 showed potent cytotoxicity against five human tumor cells, while compounds 1 and 6 did not. In the case of compounds 1 and 2, 3-oxo sterol derivatives, compound 1 was inactive, but compound 2 showed potent cytotoxicity. In addition, compound 5 exhibited potent cytotxicity, but, compound 6 which is the 7-epimer of compound 5 was weakly active against tumor cell lines. Therefore, in the case of oxysterol derivatives, the cytotoxicity appeared to be affected by the structural differences, i.e. the configuration of hydroxyl group and the number of conjugated double bond. Taken all together, the present study isolated six sterols from S. tamariscina for the first time based on a bioassay-guided fractionation and indicated that isolated oxysterols could exhibit the cytotoxic effects against tumor cells, suggesting that S. tamariscina might be a promising candidate for the development of anticancer agents.
Similar content being viewed by others
References
Ahn, S. H., Mun, Y. J., Lee, S. W., Kwak, S., Choi, M. K., Baik, S. K., Kim, Y. M., and Woo, W. H., Selaginella tamariscina induces apoptosis via a caspase-3-mediated mechanism in human promyelocytic leukemia cells. J. Med. Food, 9, 138–144 (2006).
Akihisa, T., Hayashi, Y., Patterson, G. W., Shimizu, N., and Tamura, T., 4α-Methylvernosterol and other sterols from Vernonia anthelmintica seeds. Phytochemistry, 31, 1759–1763 (1992).
Carlo, G. D., Masclo, N., Izzo, A. A., and Capasso, F., Flavonoids: Old and new aspects of a class of natural therapeutic drugs. Life Sci., 65, 337–353 (1999).
Das, B., Venkateswarlu, Y., Srinivas, K. V. N. S., and Rama Rao, A. V., 5α-Poriferast-9(11)-en-3β-ol from the marine red alga, Gracilaria edulis. Phytochemistry, 31, 1054–1055 (1992).
Das, B. and Srinivas, K. V. N. S., Minor C29-steroids from the marine red alga, Gracilaria edulis. Phytochemistry, 31, 2427–2429 (1992a).
Das, B. and Srinivas, K. V. N. S., Dihydroxysterols from the marine red alga, Gracilaria edulis. Phytochemistry, 31, 4371–4373 (1992b).
Fukuyama, Y., Nakano, Y., Pei-Wu, G., Rui, W., Sumitomo, J., Jinxian, B., and Nakagawa, K., In vitro fibrinolytic phytosterols isolated from the roots of Spatholobus suberetus. Planta Med., 54, 34–36 (1988).
Gao, L. L., Yin, S. L., Li, Z. L., Sha, Y., Pei, Y. H., Shi, G., Jing, Y. K., and Hua, H. M., Three novel sterols isolated from Selaginella tamariscina with antiproliferative activity in leukemia cells. Planta Med., 73, 1112–1115 (2007).
Hyun, J. W., Weltin, D., Holl, V., Marchal, J., Dufour, P., Luu, B., and Bischoff, P., Cytotoxic properties of a phosphoglycoconjugated derivative of 7 beta-hydroxycholesterol upon normal and tumor cells in culture. Anticancer Res., 17, 2621–2626 (1997).
Hyun, J. W., Holl, V., Weltin, D., Dufour, P., Luu, B., and Bischoff, P., Effects of combinations of 7beta-hydroxycholesterol and anticancer drugs or ionizing radiation on the proliferation of cultured tumor cells. Anticancer Res., 22, 943–948 (2002).
Jung, H. J., Park, K., Lee, I. S., Kim, H. S., Yeo, S. H., Woo, E.-R., and Lee, D. G., S-phase accumulation of Candida albicans by anticandidal effect of amentoflavone isolated from Selaginella tamariscina. Biol. Pharm. Bull., 30, 1969–1971(2007).
Kwon, H. C., Zee, S. D., Cho, S, Y., Choi, S. U., and Lee, K. R., Cytotoxic ergosterols from paecilomyces sp. J300. Arch. Pharm. Res., 25, 851–855 (2002).
Lee, C. W., Choi, H. J., Kim, H. S., Kim, D. H., Chang, I. S., Moon, H. T., Lee, S. Y., Oh, W. K., and Woo, E.-R., Biflavonoids isolated from Selaginella tamariscina regulate the expression of matrix metalloproteinase in human skin fibroblasts. Bioorg. Med. Chem., 16, 732–738 (2008).
Lee, I. S., Nishikawa, A., Furukawa, F., Kasahara, K., and Kim, S. U., Effects of Selaginella tamariscina on in vitro tumor cell growth, p53 expression, G1 arrest and in vivo gastric cell proliferation. Cancer Lett., 144, 93–99 (1999).
Lee, J., Choi, Y., Woo, E.-R., and Lee, D. G., Isocryptomerin, a novel membrane-active antifungal compound from Selaginella tamariscina. Biochem. Biophys. Res. Commun., 379, 676–80 (2009a).
Lee, J. S., Lee, M. S., Oh, W. K., and Sul, J. Y., Fatty acid synthase inhibition by amentoflavone induces apoptosis and antiproliferation in human breast cancer cells. Biol. Pharm. Bull., 32, 1427–1432 (2009b).
Luu, B. and Moog, C., Oxysterols: biological activities and physicochemical studies. Biochimie, 73, 1317–1320 (1991).
Mahato, S. B., Nandy, A. K., and Roy, G., Triterpenoids. Phytochemistry, 31, 2199–2249 (1992).
Mahato, S. B. and Kundu, A. P., 13C-NMR spectra of pentacyclic triterpenoids - a compilation and some salient features. Phytochemistry, 37, 1517–1575 (1994).
Monks, A., Scudiero, D. A., Skehan, P., Shoemaker, R. H., Paull, K., Vistica, D. T., Hose, C., Langley, J., Cronise, P., Vaigro-Wolff, A., Gray-Goodrich, M., Campbell, H., Mayo, J., and Boyd, M. R., Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Natl. Cancer Inst., 83, 757–766 (1991).
Notaro, G., Piccialli, V., and Sica, D., New steroidal hydroxyketones and closely related diols from the marine sponge Cliona copiosa. J. Nat. Prod., 55, 1588–1594 (1992).
Paryzek, Z. and Martynow, J., Tetracyclic triterpenes. Part 16. Synthesis of 31-norcucurbitane and fusidane derivatives in the skeletal rearrangements of 9,11-epoxy-4β-demethyl-5α-lanostanes. J. Chem. Soc. Perkin Trans. I, 2, 201–207 (1996).
Pokharel, Y. R., Yang, J. W., Kim, J. Y., Oh, H. W., Jeong, H. G., Woo, E.-R., and Kang, K. W., Potent inhibition of the inductions of inducible nitric oxide synthase and cyclooxygenase-2 by taiwaniaflavone. Nitric Oxide, 15, 217–225 (2006).
Reisch, J. and Cramer, M., 7β-Hydroxycholesterol, eine ubiquitre signalsubstanz im thymusgewebe. Pharmazie, 49, 75–76 (1994).
Schroepfer, G. J. Jr., Oxysterols: modulators of cholesterol metabolism and other processes. Physiol. Rev., 80, 361–554 (2000).
Silva, G. L., Chai, H., Gupta, M. P., Farnsworth, N. R., Cordell, G. A., Pezzuto, J. M., Beecher, C. W., and Kinghorn, A. D., Cytotoxic biflavonoids from Selaginella willdenowii. Phytochemistry, 40, 129–134 (1995).
Skehan, P., Storeng, R., Scudiero, D., Monk, A., McMahon, J., Vistica, D., Warren, J., Bokesch, H., Kenny, S., and Boyd, M. R., New colorimetric cytotoxicity assay for anticancerdrug screening. J. Natl. Cancer Inst., 82, 1107–1112 (1990).
Yang, S. F., Chu, S. C., Liu, S. J., Chen, Y. C., Chang, Y. Z., and Hsieh, Y. S., Antimetastatic activities of Selaginella tamariscina (Beauv.) on lung cancer cells in vitro and in vivo. J. Ethnopharmacol., 110, 483–489 (2007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roh, E.M., Jin, Q., Jin, HG. et al. Structural implication in cytotoxic effects of sterols from Sellaginella tamariscina . Arch. Pharm. Res. 33, 1347–1353 (2010). https://doi.org/10.1007/s12272-010-0908-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-010-0908-8