Abstract
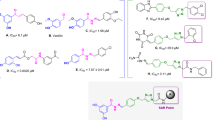
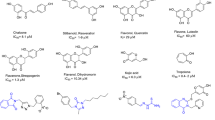
A novel 3″,4″-dihydroglabridin was successfully prepared for studying on tyrosinase inhibitory activity. The result demonstrated that 3″,4″-dihydroglabridin exhibited higher activity than glabridin (IC50 value = 11.40 μM), which is probably due to the 4-substituted resorcinol skeleton and the lacking of double bond between carbon atom 3″ and 4″ on its structure giving more conformational flexibily to interact with the enzyme more effectively. In addition, various acylated derivatives were synthesized as glabridin prodrugs. The chemical and enzymatic hydrolysis of prodrugs revealed that the diacetate ester was rapidly hydrolyzed by porcine liver esterase with the half-life of 2.36 minute, while those of the dihexanoate was 14.8 hour. Both of them were sufficiently stable in phosphate buffer, both pH 5.5 and 7.4, at 37°C with more than 15 days half-life.
Similar content being viewed by others
References
Belinky, P. A., Aviram, M., Fuhraman, B., Rosenblat, M., and Vaya, J., The antioxidative effects of the isoflavan glabridin on endogenous constituents of LDL during its oxidation. Atherosclerosis., 137, 49–61 (1998a).
Belinky, P. A., Aviram, M., Mahmood, S., and Vaya, J., Structural aspects of the inhibitory effect of glabridin on LDL oxidation. Free Radic. Biol. Med., 24, 1419–1429 (1998b).
Briton, G., The biochemistry of natural pigments. Cambridge University Press, London, (1983).
Fukai, T., Marumo, A., Kaitou, K., Kanda, T., Terada, S., and Nomura, T., Antimicrobial activity of licorice flavonoids against methicillin-resistant Staphylococcus aureus. Fitoterapia., 73, 536–539 (2002).
Fukai, T., Satoh, K., Nomura, T., and Sakagami, H., Preliminary evaluation of antinephritis and radical scavenging activities of glabridin from Glycyrrhiza glabra. Fitoterapia., 74, 624–629 (2003).
van Gelder, C. W. G., Flurkey, W. H., and Wichers, H. J., Sequence and structural features of plant and fungal tyrosinases. Phytochemistry., 45, 1309–1323 (1997).
Haraguchi, H., Yoshida, N., Ishikawa, H., Tamura, Y., Mizutani, K., and Kinoshita, T., Protection of mitochondrial functions against oxidative stresses by isoflavans from Glycyrrhiza glabra. J. Pharm. Pharmacol., 52, 219–223 (2000).
Iida, K., Hase, K., Shimomura, K., Sudo, S., Kadota, S., and Namba, T., Potent inhibitors of tyrosinase activity and melanin biosynthesis from Rheum officinale. Planta Med., 61, 425–428 (1995).
Kim, Y. J. and Uyama, H., Tyrosinase inhibitors from natural and synthetic sources: structure, inhibition mechanism and perspective for the future. Cell. Mol. Life Sci., 62, 1707–1723 (2005).
Kinoshita, T., Kajiyama, K., Hiraga, Y, Takahashi, K., Tamura, Y., and Mizutani, K., Isoflavan derivatives from Glycyrrhiza glabra (licorice). Heterocycles., 43, 581–588 (1996).
Likhitwitayawuid, K. and Sritularak, B., A new dimeric stilbene with tyrosinase inhibitory activity from Artocarpus gomezianus. J. Nat. Prod., 64, 1457–1459 (2001).
Nerya, O., Vaya, J., Musa, R., Izrael, S., Ben-Arie, R., and Tamir, S., Glabrene and isoliquiritigenin as tyrosinase inhibitors from licorice roots. J. Agric. Food Chem., 51, 1201–1207 (2003).
Redden, P. R., Melanson, R. L., Douglas, J. -A. E., and Dick, A. J., Acyloxymethyl acidic drug derivatives: in vitro hydrolytic reactivity. Int. J. Pharm., 180, 151–160 (1999).
Seo, S. Y., Sharma, V. K., and Sharma, N., Mushroom tyrosinase: recent prospects. J. Agric. Food Chem., 51, 2837–2853 (2003).
Shimizu, K., Kondo, R., and Sakai, K., Inhibition of tyrosinase by flavonoids, stilbenes and related 4-substituted resorcinols: structure-activity investigations. Planta Med., 66, 11–15 (2000).
Shimizu, K., Kondo, R., Sakai, K., Lee, S. H., and Sato, H., The inhibitory components from Artocarpus incisus on melanin biosynthesis. Planta Med., 64, 408–412 (1998).
Somjen, D., Knoll, E., Vaya, J., Stern, N., and Tamir, S., Estrogen-like activity of licorice root constituents: glabridin and glabrene, in vascular tissues in vitro and in vivo. J. Steroid Biochem. Mol. Biol., 91, 147–155 (2004).
Tamir, S., Eizenberg, M., Somjen, D., Stern, N., Shelach, R., Kaye, A., and Vaya, J., Estrogenic and antiproliferative properties of glabridin from licorice in human breast cancer cells. Cancer Res., 60, 5704–5709 (2000).
Wang, B., Zhang, H., Zheng, A., and Wang, W., Coumarinbased prodrugs. part 3: structure effects on the release kinetics of esterase-sensitive prodrugs of amines. Bioorg. Med. Chem., 6, 417–426 (1998).
Xu, C. R., He, H. T., Song, X., and Siahaan, T. J., Synthesis and comparison of physicochemical, transport, and antithrombic properties of a cyclic prodrug and the parent RGD peptidomimetic. Tetrahedron., 59, 2861–2869 (2003).
Yokota, T., Nishio, H., Kubota, Y., and Mizoguchi, M., The inhibitory effect of glabridin from licorice extracts on melanogenesis and inflammation. Pigment Cell Res., 11, 355–361 (1998).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jirawattanapong, W., Saifah, E. & Patarapanich, C. Synthesis of glabridin derivatives as tyrosinase inhibitors. Arch. Pharm. Res. 32, 647–654 (2009). https://doi.org/10.1007/s12272-009-1501-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-009-1501-x