Abstract
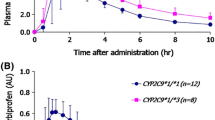
The genetically polymorphic CYP2C9 metabolizes many non-steroidal anti-inflammatory agents, including naproxen. This study examined the effects of a CYP2C9 genetic polymorphism on the pharmacokinetics of naproxen in Korean subjects. Twenty healthy male subjects carrying a CYP2C9*1/*1 (n=14) or CYP2C9*1/*3 (n=6) polymorphism were enrolled. After a single-dose of 275 mg naproxen Na, blood samples were collected at various times over a 72 h period and the plasma naproxen concentration was measured. The plasma concentration of naproxen was determined by HPLC analysis with UV detection, and the pharmacokinetic parameters were calculated. The mean plasma concentration-time profiles of naproxen in the CYP2C9*1/*3 and CYP2C9*1/*1 individuals were similar. There were no significant differences in the pharmacokinetics of naproxen between CYP2C9*1/*1 and CYP2C9*1/*3 genotypes. The AUC0-∞ (p = 0.759) and oral clearance (p = 0.823) of naproxen were also similar in individuals with CYP2C9*1/*3 and CYP2C9*1/*1. Overall, a genetic polymorphism of CYP2C9 does not significantly affect the pharmacokinetics of naproxen. Therefore, naproxen does not require a dose adjustment for individuals with the CYP2C9*1/*3 genotype.
Similar content being viewed by others
References
Andersen, J. V. and Hansen, S. H., Simultaneous quantitative determination of naproxen, its metabolite 6-O-desmethylnaproxen and their five conjugates in plasma and urine samples by high-performance liquid chromatography on dynamically modified silica. J. Chromatogr., 577, 325–333 (1992).
Bae, J. W., Kim, H. K., Kim, J. H., Yang, S. I., Kim, M. J., Jang, C. G., Park, Y. S., and Lee, S. Y., Allele and genotype frequencies of CYP2C9 in a Korean population. Br. J. Clin. Pharmacol., 60, 418–422 (2005).
Becker, M. L, Visser, L. E., Trienekens, P. H., Hofman, A., van Schaik, R. H., and Stricker, B. H., Cytochrome P450 2C9 *2 and *3 polymorphisms and the dose and effect of sulfonylurea in type II diabetes mellitus. Clin. Pharmacol. Ther. 83, 288–292 (2008).
Chen, G., Jiang, S., Mao, G., Zhang, S., Hong, X., Tang, G., Li, Z., Liu, X., Zhang, Y., Xing, H., Wang, B., Yu, Y., and Xu, X., CYP2C9 Ile359Leu polymorphism, plasma irbesartan concentration and acute blood pressure reductions in response to irbesartan treatment in Chinese hypertensive patients. Methods Find Exp. Clin. Pharmacol. 28, 19–24 (2006).
DeArmond, B., Francisco, C. A., Lin, J. S., Huang, F. Y., Halladay, S., Bartziek, R. D., and Skare, K. L., Safety profile of over-the-counter naproxen sodium. Clin. Ther., 17, 587–601 (1995).
Dollery, C., Therapeutic Drugs, 2nd ed. Churchill Livingstone, London, N31–N36 (1999).
Foster, R. T., Jamali, F., Russell, A. S., and Alballa, S. R., Pharmacokinetics of ketoprofen enantiomers in healthy subjects following single and multiple doses. J. Pharm. Sci., 77, 70–73 (1988).
Hamman, M. A., Haehner-Daniels, B. D., Wrighton, S. A., Rettie, A. E., and Hall, S. D., Stereoselective sulfoxidation of sulindac sulfide by flavin-containing monooxygenases. Comparison of human liver and kidney microsomes and mammalian enzymes. Biochem. Pharmacol., 60, 7–17 (2000).
Human Cytochrome P450 (CYP) Allele Nomenclature Committee. Available from www.cypalleles.ki.se/cyp2c9.htm. Accessed August 11, 2008.
Kiang, C. H., Lee, C., and Kushinsky, S., Isolation and identification of 6-desmethylnaproxen sulfate as a new metabolite of naproxen in human plasma. Drug Metab. Dispos. 17, 43–48 (1989).
Kidd, R. S., Straughm, A. B., Meyer, M. C., Blaisdell, J., Goldstein, J. A., and Dalton, J. T., Pharmacokinetics of chlorpheniramine, phenytoin, glipizide and nifedipine in an individual homozygous for the CYP2C9*3 allele. Pharmacogenetics 9, 71–80 (1999).
Lee, C. R., Pieper, J. A., Frye, R. F., Hinderliter, A. L., Blaisdell, J. A., and Goldstein, J. A., Differences in flurbiprofen pharmacokinetics between CYP2C9*1/*1, *1/*2, and *1/*3 genotypes. Eur. J. Clin. Pharmacol. 58, 791–794 (2003).
Lee, Y. J., Kim, Y. G., Lee, M. G., Chung, S. J., Lee, M. H., and Shim, C. K., Analysis of bioequivalence study using log-transformed model. Yakhakhoeji 44, 308–314 (2000).
Liu, Y. L., Zhang, W., Tan, Z. R., Ouyang, D. S., Luo, C. H., Liu, Z. Q., Qiu, Y., Chen, Y., He, Y. J., Zhou, G., and Zhou, H. H., Effect of the CYP2C9*3 allele on lornoxicam metabolism. Clin. Chim. Acta. 364, 287–291 (2006).
Liu, Y. L., Zhang, W., Tan, Z. R., Ouyang, D. S., Luo, C. H., Liu, Z. Q., Qiu, Y., Chen, Y., He, Y. J., Zhou, G., and Zhou, H. H., Effect of the CYP2C9*3 allele on lornoxicam metabolism. Clin. Chim. Acta 364, 287–291 (2006).
Miner, J. O. and Birkett, D. J., Cytochrome P4502C9: an enzyme of major importance in human drug metabolism. Br. J. Clin. Pharmacol., 45, 525–538 (1998).
Miners, J. O., Coulter, S., Tukey, R. H., Veronese, M. E., and Birkett, D. J., Cytochrome P450, 1A2, and 2C9 are responsible for the human hepatic o-desmethylation of Rand S-naproxen, Biochem. Pharmacol. 51, 1003–1008 (1996).
Myrand, S., Sekiguchi, K., Man, M., Lin, X., Tzeng, R. Y., Teng, C. H., Hee, B., Garrett, M., Kikkawa, H., Lin, C. Y., Eddy, S. M., Dostalik, J., Mount, J., Azuma, J., Fujio, Y., Jang, I. J., Shin, S. G., Bleavins, M. R., Williams, J. A., Paulauskis, J. D., and Wilner, K. D., Pharmacokinetics/Genotype Associations for Major Cytochrome P450 Enzymes in Native and First- and Third-generation Japanese Populations: Comparison With Korean, Chinese, and Caucasian Populations. Clin. Pharmacol. Ther., 84, 347–361 (2008).
Perini, J. A., Vianna-Jorge, R., Brogliato, A. R., and Suarez-Kurtz, G., Influence of CYP2C9 genotypes on the pharmacokinetics and pharmacodynamics of piroxicam. Clin. Pharmacol. Ther., 78, 362–369 (2005).
Rendic, S. and di Carlo, F. J., Human cytochrome P450 enzymes: a status report summarizing their reactions, substrates, inducers, and inhibitors. Drug Metab. Rev., 29, 413–580 (1997).
Rodrigues, A. D., Impact of CYP2C9 genotype on pharmacokinetics: are all cyclooxygenase inhibitors the same? Drug Metab. Dispos., 33, 1567–1575 (2005).
Rodrigues, A. D., Kukulka, M. J., Roberts, E. M., Ouellet, D., and Rodgers, T. R., [O-Methyl 14C] naproxen o-desmethylase activity in human liver microsomes: evidence for the involvement of cytochrome P4501A2 and P4502C9/10, Drug Metab. Dispos., 24, 126–136 (1996).
Sandberg, M., Yasar, U., Strömberg, P., Höög, J. O., and Eliasson, E., Oxidation of celecoxib by polymorphic cytochrome P450 2C9 and alcohol dehydrogenase. Br. J. Clin. Pharmacol., 54, 423–429 (2002).
Suzuki, K., Yanagawa, T., Shibasaki, T., Kaniwa, N., Hasegawa, R., and Tohkin, M., Effect of CYP2C9 genetic polymorphisms on the efficacy and pharmacokinetics of glimepiride in subjects with type 2 diabetes. Diabetes Res. Clin. Pract., 72, 148–154 (2006).
Thomson, G. F. and Collins, G. M., Urinary metabolic profiles for choosing test animals for chronic toxicity studies: application to naproxen. J. Pharm. Sci., 62, 937–941 (1973)
Tracy, T. S., Marra, S., Wrighton, S. A., Gonzalez, F. J., and Korzekwa, K.R., Involvement of multiple cytochrome P450 isoforms in naproxen O-desmethylation, Eur. J. Clin. Pharmacol., 52, 293–298 (1997).
Vianna-Jorge, R., Perini, J. A., Rondinelli, E., and Suarez-Kurtz, G., CYP2C9 genotypes and the pharmacokinetics of tenoxicam in Brazilians. Clin. Pharmacol. Ther., 76, 18–26 (2004).
Xie, H. G., Prasad, H. C., Kim, R. B., and Stein, C. M., CYP2C9 allelic variants: ethnic distribution and functional significance. Adv. Drug Deliver. Rev., 54, 1257–1270 (2002).
Yoon, Y. R., Shon, J. H., Kim, M. K., Lim, Y. C., Lee, H. R., Park, J. Y., Cha, I. J., and Shin J. G., Frequency of cytochrome P450 2C9 mutant alleles in a Korean population. Br. J. Clin. Pharmacol., 51, 277–280 (2001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bae, JW., Kim, JH., Choi, CI. et al. Effect of CYP2C9*3 allele on the pharmacokinetics of naproxen in Korean subjects. Arch. Pharm. Res. 32, 269–273 (2009). https://doi.org/10.1007/s12272-009-1232-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-009-1232-z