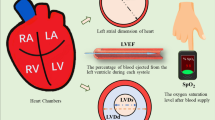
Abstract
Heart failure (HF) is defined as the inability of the heart to meet body oxygen demand requiring an elevation in left ventricular filling pressures (LVP) to compensate. LVP increase can be assessed in the cardiac catheterization laboratory, but this procedure is invasive and time-consuming to the extent that physicians rather rely on non-invasive diagnostic tools. In this work, we assess the feasibility to develop a novel machine-learning (ML) approach to predict clinically relevant LVP indices. Synchronized invasive (pressure–volume tracings) and non-invasive signals (ECG, pulse oximetry, and cardiac sounds) were collected from anesthetized, closed-chest Göttingen minipigs. Animals were either healthy or had HF with reduced ejection fraction and circa 500 heartbeats were included in the analysis for each animal. The ML algorithm showed excellent prediction of LVP indices estimating, for instance, the end-diastolic pressure with a R2 of 0.955. This novel ML algorithm could assist clinicians in the care of HF patients.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heart failure (HF) is defined as the inability of the heart to meet body oxygen demand at either rest or exercise requiring an increase of cardiac filling pressure in order to meet that necessity [1]. It is a clinical syndrome with symptoms or signs caused by structural and functional cardiac abnormalities confirmed by elevated natriuretic peptide levels and/or objective evidence of pulmonary or systemic congestion [2]. Central to diagnosis are also specific thresholds of ventricular function such as left ventricular ejection fraction (LVEF).
Based on the latter parameter, HF has been subclassified into 3 categories: HF with preserved ejection fraction (LVEF ≥ 50%), HF with midrange ejection fraction (LVEF 41%-49%) and HF with reduced ejection fraction (HFrEF, LVEF < 40%) [3]. However, classifying HF on the basis of EF alone has its limitations as this parameters mostly reflects LV geometry rather than systolic function [4] and, by itself, cannot recapitulate the underlying pathophysiology of the disease [5]. Therefore, LV filling pressures plays a central role in the definition of the pathology (LV end-diastolic pressure, LV EDP > 15 mmHg at rest as a diagnostic cutoff for HF [6]), but they can only be reliably assessed via cardiac catheterization, which is invasive, time-consuming, associated with patient’s pain, and periprocedural complications [7, 8].
Regarding the bedside clinical examination, physicians rather rely on a combination of non-invasive assessments of cardiac function. However, available non-invasive methodologies such as ECG, pulse oximetry, cardiac sounds, or ultrasound imaging, have their own technical limitations [9,10,11], thus making the pursue of new approaches highly desirable [12].
Among the innovative approaches, a promising niche belongs to artificial intelligence (AI) who has the potential to assist clinicians in the decision-making process. Much of its merit is related to its ability to handle a vast amount of complex data and then derive novel and useful insights into clinical practice. Machine learning (ML) is an AI technique which can be designed to process data that it can quickly learn from [13]. To highlight its relevance, for instance, the U.S.A. Food and Drug Administration issued the AI/ML Action Plan (https://www.fda.gov/medical-devices/software-medical-device-samd/artificial-intelligence-and-machine-learning-software-medical-device), which aims to integrate ML and AI into medical device software.
In this work, we set out to investigate the feasibility of a ML approach to non-invasively predict clinically relevant hemodynamic indices of LV function such as: end-diastolic pressure (EDP), end-systolic pressure (ESP), and maximum rate of pressure rise (dPdtmax). In order to build up our ML model, we have invasively measured the above-mentioned hemodynamic indices in a clinically relevant, tachypacing-induced, porcine model of HFrEF [14] as well as in a healthy control. ML features (i.e. predictors) were extracted from simultaneously acquired non-invasive signals such as ECG, pulse oximetry and cardiac sounds.
Materials and Methods
Porcine Model of Heart Failure with Reduced Ejection Fraction
The experimental protocol was approved by the local bioethics committee of Berlin, Germany (G 0064/19), and conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health (NIH Publication No. 85–23, revised 1996). The 2 animals included in the present proof-of-concept work were part of an already published study in which HF was induced in minipigs (Ellegaard Göttingen Minipigs, Dalmose, DK) by continuous right ventricular (RV) pacemaker stimulation (tachypacing) [14, 15]. Briefly, minipigs were exposed to incremental ventricular tachycardia for a total of 6 weeks, with 180 beats/min for 2 weeks followed by 200 beats/min for further 4 weeks. Already after 4 weeks of pacing, chronic compensated HF with highly depressed LVEF (< 40%) was induced in all animals, with an average of 22 ± 6% at 6 weeks. Healthy Göttingen minipigs were enrolled as control group [15]. At 6 weeks, final invasive LV pressure–volume measurements and right heart catheterization were performed. Animals were acutely instrumented closed-chest under fluoroscopic guidance with a pulmonary artery flotation catheter (Swan Ganz, paediatric, 5F, Edwards Lifesciences connected to Vigilance II, Edwards Lifesciences, Irvine, CA) and a LV conductance catheter (5F, 12 electrodes, 7-mm spacing; MPVS Ultra, Millar Instruments, Houston, TX) as previously described [16].
Acquisition of Signals
In the final invasive measurement, the following signals were acquired via LabChart 8 software (ADInstruments Ltd, Oxford, UK) at a sampling frequency of 1 kHz in anesthetized, closed-chest, Göttingen minipigs:
-
1) ECG signal [V], using a standard 3-lead ECG;
-
2) LVP signal [mmHg], which is the LV pressure derived by the conductance catheter fluoroscopically positioned into the LV apex;
-
3) MIC signal [mmHg], namely, the phonocardiogram obtained via a microphone (MLT201 Cardio Microphone, ADInstruments) attached to the thoracic skin at the level of LV apex;
-
4) POX signal [mmHg], that is, the pulse oximetry acquired with a tail-cuff.
In-Vivo Experimental Protocol and Preliminary Processing of the Signals
The acquisition of the signals started at spontaneous heart rate (HR), followed by incremental RV pacing rates with steps of 20 beats/min up to a maximum of 160 beats/min, for a total of 5 measurement steps (spontaneous, 100, 120, 140 and 160 beats/min). These steps are routinely performed for a complete hemodynamic assessment of LV function during progressively decreasing diastolic times in order to simulate the HR increase during exercise or stress. For further analysis, each recorded step was cut by selecting a duration of at least 3 min of stable hemodynamic conditions, i.e. with less than 10% variation in systemic pressure. In particular, using a custom-made script written in MATLAB® language (The MathWorks, Inc., Natick, MA), the HR was detected via ECG analysis, then the MIC signal was up- and down-enveloped, and the difference between the two envelopes was calculated for each cut segment (i.e. measurement step). The envelope of an oscillating signal is a smooth curve outlining its extremes, in our case the local maxima and the local minima of the MIC signal; the envelope, thus, generalizes the concept of a constant amplitude into an instantaneous amplitude. The difference between the upper and lower envelopes is called MED signal [mmHg] (Microphone Envelope Difference) and we bandpass-filtered it by passing only the HR previously detected in its specific segment. In conclusion, we have obtained five MED signals, one for each pacing step.
As displayed in Figs. 1 and 2, the local minima of the MED sinus-like signal were useful, for each couple of consecutive heart cycles, to approximate the temporal boundary between systole and diastole without using the LVP signal (Figs. 1 and 2). More specifically, the local minima of the MED sinus-like signal identify the peaks of the LVP signal and the T wave on the ECG signal.
Synchronization of ECG, left ventricular pressure, cardiac sounds and pulse oximetry signals in Göttingen minipigs. Signal synchronization with ECG in black, left ventricle pressure (LVP) in red, cardiac sounds (MIC) in green, and pulse oximetry (POX) in blue. All signals were recorded, at a sampling frequency of 1 kHz, in 1 un-paced healthy swing (panel a) and 1 un-paced HFrEF swine (panel b), both with a spontaneous heart rate of circa 80 beats/min. The Microphone Envelope Difference (MED), which is the difference between the upper and lower envelopes of the MIC signals, is displayed in pink. The local minima of the MED sinus-like signal were useful, for each couple of consecutive heart cycles, to approximate the temporal boundary between systole and diastole without using the LVP signal. Note that the cardiac sounds are louder in the un-paced heart failure swine
Representative Microphone Envelope Difference (MED) in an un-paced heart failure Göttingen minipig. The graphs highlight that the local minima of the MED sinus-like signal can be used to approximate the temporal boundary between systole and diastole for each couple of consecutive heart cycles (panel a and b). In detail, the local minima of the MED signal identify the peaks of the LVP signal
Features Extraction for the Prediction of Left Ventricle Indices
The main aim of our study was to define features (i.e., predictors) extracted from cardiac sounds, ECG, and pulse oximetry in order to predict via linear regression the following LV pressure indices: EDP [mmHg], ESP [mmHg], and dPdtmax [mmHg/s].
Using a custom-made script written in MATLAB® language, via ECG analysis and by the local minima of the MED sinus-like signal, each heartbeat was identified and segmented into diastole and systole for the single-beat analysis of the following indices:
-
i)
EDP, ESP, dPdtmax (parameters to be predicted) calculated from the invasively assessed LVP signal;
-
ii)
the multiplicative inverse of the pulse transit time (invPTT) calculated from the ECG and the pulse oximetry signals.
The invPTT has been already described in literature as a good predictor of systemic blood pressure as, for instance, Wibmer et al. [17] found a clear negative correlation between pulse transit time (PTT) and mean systolic blood pressure, as previously reviewed [18].
In addition, for each heartbeat and separately for diastole and systole, we analyzed the melody spectrum (MEL) of the MIC signal, from which we computed two sets of predictors employing the MATLAB® Audio Toolbox™ (The MathWorks, Inc., Natick, MA):
-
i)
set A comprising spectral flux [19], spectral kurtosis [20,21,22,23], spectral skewness [20,21,22], spectral slope [24, 25];
-
ii)
set B including the 13 MEL frequency cepstral coefficients in 2nd derivative (delta-delta-MFCCs) [26,27,28,29,30].
The rationale of calculating both time and frequency features (invPTT and MEL predictors, respectively) is that similar combinations of “time plus frequency” have been crucial to achieve improved results in works about heart sounds. As an overall strategy, the systolic sounds were employed to predict the precedent EDP and the contemporary dPdtmax, whereas the diastolic sounds were employed to predict the precedent ESP.
Results
In this preclinical feasibility study, circa 500 cardiac cycles from each minipig (1 HFrEF and 1 healthy control) were included in the final analysis. Table 1 summarizes the baseline hemodynamic characteristics of the two animals on the day of the final invasive LV pressure–volume measurements. As expected, the HFrEF animal showed a highly depressed ejection fraction (LV EF 16%), while the healthy control had a preserved LV EF of 50%. Stroke volume and cardiac output were severely depressed in HFrEF, with LV EDP being above the diagnostic cutoff for HF (> 15 mmHg).
Prediction of LVP Indices
In order to test the validity of the extracted features in predicting LV pressure indices, we tested the prediction of the combination of the above-described MEL features (sets A and B) with the calculated invPTT against invPTT alone. For instance, Fig. 3a shows the linear regression analysis where the “whole model” (i.e. all the predicting features condensed in their linear combination) predicts the LV EDP in a healthy animal at a pacing stimulation of 100 bpm. Figure 3b corresponds to the linear regression analysis where the whole model predicts the LV EDP in a HFrEF animal at a spontaneous HR of circa 80 bpm.
Representative linear regression analysis to predict the invasively measured LV EDP. The regressions are calculated at a pacing stimulation of 100 bpm (panel a, healthy animal, R2 = 0.694 and RMSE = 0.82 mmHg, with a relative error of less than 5%) and at a spontaneous HR of 80 bpm (panel b, HFrEF animal, R2 = 0.955 and RMSE = 0.3 mmHg, with a relative error of less than 2%). The whole model (x axis) is the linear combination of the MEL features of the cardiac sounds (set A and set B) plus the invPTT. The blue crosses represent the analyzed heartbeats, the red line is the linear regression fit to predict the LV EDP, and the red dotted curves are the 95% confidence bounds for the linear regression
In particular for the HFrEF, we observed an excellent R2 (coefficient of determination) of 0.955 and a small RMSE (root mean square error) of ca. 0.3 mmHg: in other words, we committed a relative error of less than 2% in the indirect, non-invasive estimation of the LV pressure at the end of the diastole.
Table 2 summarizes the results for the prediction of LV EDP, LV ESP and LV dPdtmax for both the HFrEF and the healthy animals in spontaneous HR. Taken together, the MEL features of the cardiac sounds (set A plus set B) combined with the calculated invPTT showed a very good prediction skill.
Overall, the linear combination of MEL features of the cardiac sounds with the invPTT performed better in predicting the invasively measured LV pressure indices compared to invPTT alone in both healthy (LV EDP R2 0.189 vs 0.003, LV ESP R2 0.230 vs 0.006, LV dPdtmax R2 0.340 vs 0.000; MEL + invPTT vs invPTT, respectively) and HFrEF pigs (LV EDP R2 0.955 vs 0.776; LV ESP R2 0.944 vs 0.897; LV dPdtmax R2 0.934 vs 0.889; MEL + invPTT vs invPTT, respectively).
Prediction of LVP Indices During the RV Pacing Steps
Tables 3 and 4 display the prediction skill of our linear regression model during RV pacing up to 160 beats/min for both HFrEF and healthy pigs. The RV pacing steps are routinely performed for the hemodynamic assessment of LV function during progressively decreasing diastolic times. Overall, the performance of our model to predict LVP indices was worsened by pacing at higher heart rates, in particular at a pacing rate of 140 bpm, probably because of the progressive shortening of the diastolic time. As it occurred during spontaneous beating, the combined use of MEL features of the cardiac sounds (set A plus set B) with the invPTT performed better than using invPTT alone.
Discussion
This study represents a proof-of-concept aiming to assess the feasibility of a ML model to predict physiologically relevant indices of LV pressure by employing a combination of non-invasive, inexpensive, and routinely applied clinical methods. Specifically, synchronized invasive (LV pressure–volume signals) and non-invasive signals (ECG, pulse oximetry and phonocardiogram via cardiac microphone) were collected from anesthetized, closed-chest Göttingen minipigs. Animals were either healthy or had HF with reduced ejection fraction induced via right ventricular tachypacing over 6 weeks. A MATLAB® environment was used to extract a set of features from cardiac sounds, ECG, and pulse oximetry signals in order to predict the LV EDP, ESP, and dPdtmax via linear regression analysis. A total of circa 500 heart cycles per animal were included in the analysis. The developed model was able to accurately predict, for instance in the HFrEF animal, the invasively measured LV EDP with a R2 of 0.955 and root mean square error of 0.3 mmHg, namely, with a relative error of less than 2% in the indirect estimation of LV EDP. [31].
Role of Left Ventricular Filling Pressure Assessment in Heart Failure
The assessment of increased LV filling pressure plays a central role in the diagnosis of HF, and LV EDP has been shown to be an important prognostic predictor in chronic stable HF patients [6]. Unfortunately, LV EDP can only be reliably measured by cardiac catheterization, which is invasive, time-consuming, and associated with risk of infection, blood clotting or embolism. Hence, clinicians seek to use non-invasive assessments such as ultrasound imaging or circulating biomarkers, which are not exempted from numerous technical limitations [12]. Therefore, a non-invasive, inexpensive, and rapid estimation of the LV filling pressure, as the one reported in this work, would be beneficial for the patient’s care. Contrary to other studies exploiting the phonocardiogram [32,33,34], a strength of our work consisted in using catheterization, being the gold standard for pressure assessment [4], in the development of the machine learning approach.
Animal Models of Heart Failure and Hemodynamic Derangements
In the past decades, several animal models recapitulating the clinical phenotype of HFrEF patients’ subgroups have been established [35]. In particular, the myocardial infarction and chronic rapid pacing models are most commonly reported in literature. In the first model, an acute myocardial ischemia is induced and is expected to lead to chronic HF over time. For instance, it can be achieved by coronary artery ligation or microembolization. This approach has the benefit to model one of the most frequent underlying causes of HFrEF, but comes with risks of lethal arrhythmias, low reproducibility and often leads to moderate LV dysfunction [35]. The second model has been first described in the 1970s [36] and is based on pacing the heart at a rate 2–4 fold higher than physiological HR. Since then, it has become the gold standard of HFrEF with loss of intrinsic myocardial contractility, dilated ventricles, and reduced contractile reserve to inotropic challenge [14]. This model offers the advantage of high reproducibility and strong LV dysfunction and comes with the typical hemodynamic and neurohumoral alterations observed in HF patients [35]. Therefore, in the past decades, it has been employed in numerous translational studies and also recently by our group to investigate HF development and novel therapeutic approaches [15].
Machine Learning to Predict Left Ventricular Pressure Indices
Our ML approach employs the linear regression model, in which LV EDP, ESP, and dPdtmax are the dependent variables to be predicted, and a set of 18 features, calculated from the ECG, POX, and MIC signals, are the independent variables. In detail, one feature is the multiplicative inverse of pulse transit time (invPTT), which was calculated from the ECG and POX signals. This parameter is considered a good predictor of systemic blood pressure as, for instance, Wibmer et al. [17] found a clear negative correlation between PTT and the systemic blood pressure. The other 17 features were calculated from the MEL spectrum of the MIC signal.
In the reported model of HFrEF, our ML approach produced worse predictions when invPTT has been used alone. This outcome is in line with recent evidence suggesting that combinations of temporal and frequency features (invPTT and MEL predictors, respectively) have improved the results in studies employing the heart sounds [28,29,30]. Indeed, we have achieved a far better prediction using the set of 18 features at each step of our frequency–response protocol for both healthy and unhealthy animals (Tables 2, 3, and 4).
Limitations
As a major limitation to the study, we recorded the required signals for the development of the ML approach in only 2 animals that were part of a trial with already extensive investigations. However, our ML model was built to predict the LV hemodynamic indices for each cardiac cycle (circa 500 heartbeats analyzed per animal) and independently from the health status of the subject. As this might help with the issue of sample size, it also poses a problem in the variability of our dataset as the physiological beat-to-beat variability might be limited. Therefore, a larger study to confirm this initial proof-of-concept is warranted. Finally, these experiments were performed under anaesthesia, whereas, hypothesizing its application in the clinic, the ML algorithm will be applied in chronic stable HF patients.
Conclusions
In summary, the newly developed machine-learning approach allows for excellent prediction of LV pressure indices. This algorithm could significantly advance both diagnostics and care of HF patients, allowing monitoring and therapy titration according to the non-invasively measured LV end-diastolic pressure.
Data Availability
Data can be provided upon formal request.
Abbreviations
- HF:
-
Heart Failure
- LVEF:
-
Left Ventricular Ejection Fraction
- HFrEF:
-
Heart Failure with reduced Ejection Fraction
- AI:
-
Artificial Intelligence
- ML:
-
Machine Learning
- EDP:
-
End-Diastolic Pressure
- ESP:
-
End-Systolic Pressure
- dPdtmax :
-
Maximum rate of pressure rise
- RV:
-
Right Ventricle
- LVP:
-
Left Ventricular Pressure signal
- MIC:
-
Microphone signal
- POX:
-
Pulse Oximetry signal
- HR:
-
Heart Rate
- MED:
-
Microphone Envelope Difference signal
- invPTT:
-
Inverse of Pulse Transit Time
- MEL:
-
Melody spectrum
References
McDonagh TA, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–726.
Murphy SP, Ibrahim NE, Januzzi JL Jr. Heart Failure With Reduced Ejection Fraction: A Review. JAMA. 2020;324(5):488–504.
Bozkurt B, et al. Universal Definition and Classification of Heart Failure: A Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail. 2021;27:387–413.
Abawi D, et al. The non-invasive assessment of myocardial work by pressure-strain analysis: clinical applications. Heart Fail Rev. 2022;27(4):1261–79.
Lam CSP, Yancy C. Universal Definition and Classification of Heart Failure: Is It universal? Does It Define Heart Failure? J Card Fail. 2021;27(5):509–11.
Reddy YNV, El-Sabbagh A, Nishimura RA. Comparing Pulmonary Arterial Wedge Pressure and Left Ventricular End Diastolic Pressure for Assessment of Left-Sided Filling Pressures. JAMA Cardiol. 2018;3(6):453–4.
Manda YR, Baradhi KM. Cardiac catheterization risks and complications. 2023 Jun 5. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024.
Al-Hijji MA, et al. Safety and Risk of Major Complications With Diagnostic Cardiac Catheterization. Circ Cardiovasc Interv. 2019;12(7):e007791.
Gurun Kaya A, et al. Is pulse oximeter a reliable tool for non-critically ill patients with COVID-19? Int J Clin Pract. 2021;75(12):e14983.
Grant MD, et al. Transthoracic Echocardiography: Beginner’s Guide with Emphasis on Blind Spots as Identified with CT and MRI. Radiographics. 2021;41(4):1022–42.
Malik SB, et al. Transthoracic Echocardiography: Pitfalls and Limitations as Delineated at Cardiac CT and MR Imaging. Radiographics. 2017;37(2):383–406.
Faragli A, et al. The role of non-invasive devices for the telemonitoring of heart failure patients. Heart Fail Rev. 2021;26(5):1063–80.
Sevakula RK, et al. State-of-the-Art Machine Learning Techniques Aiming to Improve Patient Outcomes Pertaining to the Cardiovascular System. J Am Heart Assoc. 2020;9(4):e013924.
Powers JC, Recchia F. Canine Model of Pacing-Induced Heart Failure. Methods Mol Biol. 2018;1816:309–25.
Alogna A, et al. Lung-to-Heart Nano-in-Micro Peptide Promotes Cardiac Recovery in a Pig Model of Chronic Heart Failure. J Am Coll Cardiol. 2024;83(1):47–59.
Alogna A, et al. Inotropic Effects of Experimental Hyperthermia and Hypothermia on Left Ventricular Function in Pigs-Comparison With Dobutamine. Crit Care Med. 2016;44(3):e158–67.
Wibmer T, et al. Pulse transit time and blood pressure during cardiopulmonary exercise tests. Physiol Res. 2014;63(3):287–96.
Mukkamala R, et al. Toward Ubiquitous Blood Pressure Monitoring via Pulse Transit Time: Theory and Practice. IEEE Trans Biomed Eng. 2015;62(8):1879–901.
Hao Y, et al. Spectral Flux-Based Convolutional Neural Network Architecture for Speech Source Localization and Its Real-Time Implementation. IEEE Access. 2020;8:197047–58.
Tanner K, et al. Spectral moments of the long-term average spectrum: sensitive indices of voice change after therapy? J Voice. 2005;19(2):211–22.
Lowell SY, et al. Spectral- and cepstral-based measures during continuous speech: capacity to distinguish dysphonia and consistency within a speaker. J Voice. 2011;25(5):e223–32.
Colton RH, et al. Spectral moment analysis of unilateral vocal fold paralysis. J Voice. 2011;25(3):330–6.
Antoni J, Randall RB. The spectral kurtosis: application to the vibratory surveillance and diagnostics of rotating machines. Mech Syst Signal Process. 2006;20(2):308–31.
Nakamura T, Yamauchi Y, Kawahara K. Valid method to evaluate the slope of Fourier transformed spectrum for the analysis of biological rhythm fluctuation. Biomed Mater Eng. 1995;5(1):21–8.
Guzman M, et al. Comparison of Supraglottic Activity and Spectral Slope Between Theater Actors and Vocally Untrained Subjects. J Voice. 2016;30(6):767e1–8.
Bardou D, Zhang K, Ahmad SM. Lung sounds classification using convolutional neural networks. Artif Intell Med. 2018;88:58–69.
Bozkurt B, Germanakis I, Stylianou Y. A study of time-frequency features for CNN-based automatic heart sound classification for pathology detection. Comput Biol Med. 2018;100:132–43.
Nogueira DM, et al. Classifying Heart Sounds Using Images of Motifs, MFCC and Temporal Features. J Med Syst. 2019;43(6):168.
Aziz S, et al. Phonocardiogram Signal Processing for Automatic Diagnosis of Congenital Heart Disorders through Fusion of Temporal and Cepstral Features. Sensors (Basel). 2020;20(13):3790.
Khan FA, Abid A, Khan MS. Automatic heart sound classification from segmented/unsegmented phonocardiogram signals using time and frequency features. Physiol Meas. 2020;41(5):055006.
Abawi D, et al. Cardiac power output accurately reflects external cardiac work over a wide range of inotropic states in pigs. BMC Cardiovasc Disord. 2019;19(1):217.
Ogawa S, Namino F, Mori T, Sato G, Yamakawa T, Saito S. AI diagnosis of heart sounds differentiated with super stethoscope. J Cardiol. 2023;83(4):265–271. https://doi.org/10.1016/j.jjcc.2023.09.007
Reyna MA, et al. Heart murmur detection from phonocardiogram recordings: The George B. Moody PhysioNet Challenge 2022. PLOS Digit Health. 2023;2(9):e0000324.
Bachtiger P, et al. Point-of-care screening for heart failure with reduced ejection fraction using artificial intelligence during ECG-enabled stethoscope examination in London, UK: a prospective, observational, multicentre study. Lancet Digit Health. 2022;4(2):e117–25.
Charles CJ, et al. Large Animal Models of Heart Failure: Reduced vs Preserved Ejection Fraction. Animals (Basel). 2020;10(10):1906.
Coleman HN 3rd, et al. Congestive heart failure following chronic tachycardia. Am Heart J. 1971;81(6):790–8.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
LF: signal analysis, feature extraction, machine learning, writing of the original draft, manuscript editing. FPLM: interpretation of the model, writing of the original draft, manuscript editing. JÖ: design of the animal model, experimental data collection, review of the manuscript. LB: experimental data collection, review of the manuscript. AF: review and editing of the manuscript. AA: funding acquisition, conceptualization, design of the animal model. All authors have read and agreed to the published version of the manuscript.
Informed Consent
No human studies were carried out by the authors for this article.
Aimal Studies
The experimental protocol was approved by the local bioethics committee of Berlin, Germany (G 0064/19), and conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health (NIH Publication No. 85-23, revised 1996).
Sources of Funding
Grants supporting this project were PRIN project # 202232A8AN to LF from Italian Ministry of University and Research (MUR) and PRIN project # P2022B38NR to LF from Italian Ministry of University and Research (MUR). Dr. Alogna is supported by the Deutsche Forschungsgemeinschaft (DFG; CRC 1470, Z01).
Corresponding authors
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
The authors have no conflicts of interest to declare that are relevant to the content of this article.
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
The authors have no financial or proprietary interests in any material discussed in this article.
Additional information
Associate Editor Sandra Loerakker oversaw the review of this article.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fassina, L., Muzio, F.P.L., Berboth, L. et al. Prediction of Left Ventricle Pressure Indices Via a Machine Learning Approach Combining ECG, Pulse Oximetry, and Cardiac Sounds: a Preclinical Feasibility Study. J. of Cardiovasc. Trans. Res. (2024). https://doi.org/10.1007/s12265-024-10546-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12265-024-10546-2