Abstract
Cardiac power output (CPO) is a powerful predictor of adverse outcomes in heart failure (HF). However, the original formula of CPO included the difference between mean arterial pressure and right atrial pressure (RAP). The prognostic performance of RAP-corrected CPO (CPORAP) remains unknown in heart failure with preserved ejection fraction (HFpEF). We studied 101 HF patients with a left ventricular ejection fraction > 40% who had pulmonary hypertension due to left heart disease. CPORAP was significantly more discriminating than CPO in predicting outcomes (Delong test, P = 0.004). Twenty-five (24.8%) patients presented with dis-concordantly high CPORAP and low CPO when stratified by the identified CPORAP threshold of 0.547 W and the accepted CPO threshold of 0.803 W. These patients had the lowest RAP, and their cumulative incidence was comparable with those with concordantly high CPO and CPORAP (P = 0.313). CPORAP might identify patients with right ventricular involvement, thereby providing better prognostic performance than CPO in HFpEF.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The heart is a muscular pump supplying hydraulic energy to generate both flow (cardiac output [CO]) and pressure to maintain circulation. Cardiac power output (CPO), a measure of cardiac performance, is the product of simultaneously measured CO and mean arterial pressure (MAP) (namely, CPO = MAP × CO / 451) to express cardiac pump function [1]. Several studies have demonstrated that CPO is a powerful predictor of adverse clinical outcomes in heart failure with reduced ejection fraction (HFrEF) and cardiogenic shock [1,2,3]. The prognostic value of CPO measured by echocardiography has also been determined in patients with heart failure with preserved ejection fraction (HFpEF) [4]. However, the initial derivation of CPO by Tan included the difference between MAP and right atrial pressure (RAP) in the calculation, before multiplying by CO (namely, RAP-corrected CPO [CPORAP] = [MAP-RAP] × CO / 451) [5]. The RAP component has been omitted in clinical practice and research to simplify the calculation over the past decade. Recently, the original formula has been revisited by Lim, noting the overestimation of CPO without the inclusion of RAP, particularly in patients with elevated intracardiac filling pressures [6]. Two subsequent studies have demonstrated that the prognostic performance of CPORAP is superior to CPO in both acute decompensated HFrEF and cardiogenic shock [7, 8]. However, the prognostic value of CPORAP in HFpEF remains unclear. In addition, few data regarding the prognostic impact of right heart catheterization (RHC)-derived CPO and CPORAP were available in HFpEF.
Accordingly, we investigated the association of CPO and CPORAP with clinical outcomes and hypothesized that CPORAP would provide better prognostic performance than CPO in the settings of HFpEF and heart failure with mildly reduced ejection fraction.
Methods
Study Population
In this retrospective cohort study, we enrolled consecutive heart failure (HF) patients aged ≥ 18 years with suspected pulmonary hypertension (PH) from November 2013 to June 2022. Patients underwent RHC at the Heart Failure Care Unit of our hospital. Patients were included if they (1) had pulmonary arterial wedge pressure (PAWP) > 15 mmHg; (2) had mean pulmonary arterial pressure (mPAP) > 20 mmHg; (3) had left ventricular ejection fraction (LVEF) > 40% by echocardiogram (calculated by modified Simpson method); (4) had no evidence of congenital heart disease, intracardiac shunts, or moderate to severe valvular disease. Patients with other subtypes of PH (groups 1, 3, 4, and 5) were excluded [9]. All patients completed blood tests and echocardiography within 24 h after undergoing RHC. Data regarding demographics, relevant cardiovascular and comorbid conditions, HF therapies, and laboratory and echocardiographic tests were collected by qualified cardiologists. The patients were followed up by telephone or clinic visits. Clinical outcomes including death and HF rehospitalization were collected. None of the patients underwent heart transplantation during the follow-up period. The primary outcome was event-free survival. This study complied with the principles outlined in the Declaration of Helsinki and was approved by the ethics committee of our hospital. Written informed consent was obtained from all participants.
Right Heart Catheterization and Hemodynamic Assessment
RHC was performed using the Swan-Ganz catheter (Edwards Lifesciences, USA) with echocardiographic and pressure waveform guidance. After minimal sedation, echocardiography-guided catheterization was performed through the right internal jugular vein to the pulmonary artery by HF specialists. The external pressure transducer was zeroed at the mid-thoracic level in each patient, and all pressure tracings were continuously recorded and stored. Pressure measurements were recorded at end-expiration during spontaneous breathing. Cardiac output (CO) was measured using the thermodilution method. Key hemodynamic measures recorded at the time of RHC included heart rate, systolic/diastolic/mean arterial pressure (SAP/DAP/MAP), RAP, systolic/diastolic/mean pulmonary arterial pressure (s/d/mPAP), PAWP, stroke volume (SV), and CO. Systemic vascular resistance (SVR) was calculated in Wood units as (MAP − RAP) / CO. Left ventricular effective arterial elastance (LV-Ea) was calculated as 0.9 × SAP / SV. Pulmonary vascular resistance (PVR) was calculated in Wood units as (mPAP − PAWP) / CO. Pulmonary arterial compliance (PAC) was estimated as SV / (sPAP − dPAP). CPO was defined in Watt (W) units as MAP × CO / 451, and CPORAP was defined as (MAP-RAP) × CO / 451. Pulmonary artery pulsatility index was calculated as (sPAP-dPAP) / RAP.
Statistical Analysis
Categorical values were expressed as absolute numbers (percentage) and continuous variables as median (interquartile range) or mean ± standard deviation. The Shapiro-Wilk test was used to assess normality. Differences were evaluated for continuous variables by one-way analysis of variance if normally distributed, or the Mann-Whitney U test as well as the Kruskal–Wallis test if non-normally distributed, and for categorical variables using Pearson’s χ2 test or Fisher’s exact test. The receiver operating characteristic (ROC) analysis was used to calculate a precise cut-off of CPORAP (0.547 W) and CPO (0.803 W) that would best discriminate event-free survival. We assessed the ability of CPORAP and CPO to discriminate between patients who had reached the primary outcome and those who were event-free by the close of follow-up by calculating the area under the curve (AUC), and compared performance using the Delong method. In the outcome analysis, age, sex, body mass index (BMI), New York Heart Association (NYHA) functional class, LVEF, tricuspid annular plane systolic excursion (TAPSE), N-terminal pro-B-type natriuretic peptide (NT-proBNP), history of coronary artery disease, atrial fibrillation, hypertension, diabetes, hyperlipidemia, use of loop diuretic, and hemodynamic variables were selected as possible confounders of the CPORAP association and were assessed in the univariate model. The variables that remained significant at the 0.10 level in univariable analysis were considered for inclusion in the multivariate model. A forward stepwise method was used to remove variables with a P value > 0.10 and enter variables that met a 0.05 significance level for the selection of the final multivariate model. The Kaplan-Meier analysis was used to compare the different groups for the estimation of outcomes with the log-rank test. Two-sided P values of < 0.05 were considered statistically significant. SPSS Statistics 26 (IBM, USA), R version 4.0.2 (The R Foundation, Austria), and Prism 8 (GraphPad Software, USA) were used for statistical analyses.
Results
Clinical Characteristics
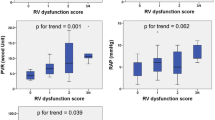
We identified 336 HF patients who underwent RHC between November 2013 and June 2022. After the screening, 101 patients met the inclusion criterion and were finally included in the analysis (Fig. 1). The median age was 58 (48–66) years and about 61% were male (Table 1). Age, sex, BMI, comorbidities, and medications did not differ between the two groups (all P > 0.05). Regarding laboratory tests and echocardiography, patients with CPORAP ≤ 0.547 W had higher serum NT-proBNP values (P = 0.001), lower LVEF (P = 0.007), and lower TAPSE (P < 0.001). As expected, patients with lower CPORAP had lower CO and SV, lower SAP and MAP, higher SVR and LV-Ea, higher mPAP and dPAP, higher PAWP, higher PVR, and lower PAC (all P < 0.05).
Clinical Outcomes Associated with CPO and CPORAP
The median duration of the follow-up period was 327 days (139–522). During the follow-up, 14 (13.9%) patients died, and 39 (38.6%) patients were rehospitalized for HF. In univariable Cox regression analysis, CPORAP was independently associated with event-free survival (HR 0.102, 95% confidence interval [CI] 0.027–0.391). After multivariate adjustment, CPORAP remained significantly associated with the primary outcome (HR 0.211, 95% CI 0.052–0.864) (Supplemental Table 1). CPO was also independently associated with event-free survival in univariable analysis (HR 0.219, 95% CI 0.075–0.644), and remained significantly associated with the primary outcome in the adjusted analyses (HR 0.270, 95% CI 0.083–0.880) (Supplemental Table 2). The Kaplan-Meier analysis and log-rank test revealed significant differences in event-free survival, whether using the optimal cut-off of 0.547 W for CPORAP (P < 0.001) or 0.803 W for CPO (P < 0.003). When further analyzing CPORAP by RAP above or below the median (12 mmHg), a significant difference in the outcome was only found for patients with RAP of more than 12 mmHg (P < 0.001) (Fig. 2). In addition, a significant difference in the outcome was also only found for patients with PVR of more than 2.2 WU (P = 0.026). However, the difference in the outcome was significant regardless of analyzing CPORAP by mPAP above or below the median (30 mmHg) (all P < 0.05) (Supplemental Fig. 1).
Survival Analysis. The Kaplan-Meier estimates of time to event-free survival stratified by CPORAP for the full cohort (A), stratified by CPO for the full cohort (B), stratified by CPORAP for patients with right atrial pressure ≤ 12 mmHg (C), stratified by CPORAP for patients with right atrial pressure > 12 mmHg (D). CPO, cardiac power output; CPORAP, right atrial pressure-corrected cardiac power output; RAP, right atrial pressure
Based on ROC analysis, CPORAP was significantly more discriminating than CPO for the prediction of event-free survival, with an AUC of 0.668 for CPORAP (95% CI: 0.563–0.772) and 0.618 for CPO (95% CI: 0.509–0.727) (Delong test, CPORAP vs. CPO: P = 0.004) (Fig. 3).
Reclassification Analyses
We further investigated the impact of reclassification by the identified CPORAP threshold of 0.547 W compared to the accepted CPO threshold of 0.803 W. A total of 42 (41.6%) patients presented with concordantly low CPORAP and CPO, 34 (33.7%) patients presented with concordantly high CPORAP and CPO, and 25 (24.8%) patients presented with dis-concordantly high CPORAP and low CPO (Fig. 4). Clinical characteristics and hemodynamic profiles for the three groups defined according to CPO and CPORAP agreement are described in Table 2. Patients in the discordant group showed intermediate serum NT-proBNP values and LVEF between patients in the concordantly high and low groups. As expected, they also exhibited intermediate CO and SV, but not MAP. Compared to the other two groups, patients in the discordant group had the lowest RAP and the highest TAPSE. In addition, there were significant differences in TAPSE, RAP, SVR, LV-Ea, PVR, and PAC between the discordant group and the concordantly low group (all P < 0.05), but there was no statistical difference in the above parameters between the discordant group and the concordantly high group (all P > 0.05).
Patients with concordantly low CPORAP and CPO had a significantly worse outcome than those with concordantly high CPORAP and CPO, as well as those with dis-concordantly high CPORAP and low CPO (all P < 0.05). There was no significant difference in the outcome between patients with concordantly high CPORAP and CPO and those with dis-concordantly high CPORAP and low CPO (P = 0.313) (Fig. 5).
Discussion
To our knowledge, this is the first report to explore the prognostic value of RHC-derived CPO and CPORAP in HF patients with an LVEF > 40%. The data in the present study demonstrate that (1) both CPO and CPORAP were associated with adverse outcomes; (2) CPORAP was superior to CPO for risk stratification; (3) the cumulative incidence of patients with low CPO reclassified as high CPORAP was comparable with that of patients with concordantly high CPO and CPORAP.
CPO is a comprehensive indicator of cardiac pump efficiency, and its prognostic effect has been well studied in patients with HF, despite the calculation of CPO in most previous studies did not incorporate RAP [1,2,3]. Since RAP is much lower than MAP in healthy people, the omission of RAP may not affect CPO calculation. However, in keeping with the concept of pressure–volume loop and Guytonian depictions of the circulatory system, RAP is an indispensable component of CPO calculation, especially when RAP is significantly elevated relative to MAP. Although the elevation in left ventricular end-diastolic pressure secondary to diastolic dysfunction is the main pathophysiological characteristic in HFpEF, the increase in RAP is also a relatively common hemodynamic profile in some patients [10, 11]. In our cohort of HF patients with an LVEF > 40%, all patients had hemodynamically defined PH, with a median mPAP of 30 mmHg and a median RAP of 12 mmHg. We demonstrated that both CPO and CPORAP were associated with adverse outcomes. These results were consistent with previous studies in patients with HFrEF or cardiogenic shock [7, 8]. Therefore, we extended on the previous studies and further found for the first time that CPORAP outperformed CPO in distinguishing patients who would experience adverse outcomes in HFpEF.
HFpEF accounts for more than half of patients with HF and frequently is associated with PH [12]. The elevation in left ventricular end-diastolic pressure and left atrial pressure are the triggers for the development of PH in HFpEF [13]. Secondary PH and pulmonary vascular disease may enhance right ventricular afterload, subsequently contributing to right ventricular dysfunction and remodeling, leading to a further increase in RAP [14, 15]. Previous studies have demonstrated that RAP could represent the cumulative cardiac burden in HFpEF [16, 17], and higher RAP is independently associated with adverse outcomes in HFpEF [17, 18]. Therefore, compared with CPO, CPORAP integrates an additional risk factor and could better identify patients with predominantly right ventricular or biventricular involvement, which could be an explanation for the better prognostic performance for adverse outcomes of CPORAP than CPO. In the present study, there was a significant difference in the outcome in patients with RAP of more than 12 mmHg after stratified by the cut-off of CPORAP, whereas patients with RAP of 12 mmHg or less were not. In addition, patients in the dis-concordantly high CPORAP and low CPO group had higher TAPSE, higher CO and SV, higher PAC, lower RAP, lower SVR and LV-Ea, and lower PVR compared with patients in the concordantly low group. These are all established markers reflective of right heart function, cardiac performance, or ventricular afterload, which may partly explain why the cumulative incidence of patients in the discordant group was significantly lower than those in the concordantly low group. Taken together, CPORAP incorporates four fundamental hemodynamic parameters (SV, heart rate, MAP, and RAP) and considers both cardiac pump function and right heart function, making it superior to CPO in risk stratification.
Indeed, HFpEF patients without right heart dysfunction can be well evaluated by the established CPO calculation. However, it is now increasingly recognized that right heart dysfunction is prevalent and contributes importantly to poor prognosis in HFpEF [19]. Moreover, several studies have identified intracardiac pressures as powerful predictors of adverse outcomes in HF [20, 21]. It is obvious that the inclusion of filling pressure into measures of cardiac function could improve prognostic performance. Therefore, compared with CPO, CPORAP could be a more comprehensive index of the global performance of the heart in HFpEF. More importantly, CPORAP could also be measured and calculated by echocardiography, as RAP could be readily estimated based on inferior vena cava diameter and its respiratory changes. Future studies are needed to validate the prognostic performance of echocardiography-derived CPORAP in HFpEF and explored whether CPORAP could be used as an indicator to evaluate the therapeutic efficacy of HFpEF.
Overall, compared with CPO, CPORAP may refine the identification of HFpEF patients at risk of adverse outcomes. Nevertheless, this study does not undermine previous reports on the predictive value of CPO in its current widely used calculations. The present study reemphasizes the concept of CPO and calls for further utilization and validation of its original derivation (CPORAP) in more clinical studies, especially with the increasing importance of right heart function in the assessment of HFpEF [22].
Limitations
Several limitations in the present study should be noted. First, this is a retrospective, single-center study with a relatively small number of patients in our cohort. However, we tried our best to ensure the accuracy of the available data. In addition, the study results are consistent with previous studies in patients with HFrEF and are supported by pathophysiological rationale. Second, all HFpEF patients in our cohort had PH. Considering that patients with PH are more likely to present with right heart dysfunction and elevated RAP, selection bias might exist in our research. Third, RHC is not a necessary diagnostic procedure for HFpEF, especially in those patients who do not have suspected PH or who have already been diagnosed with HFpEF by routine examination. The results may, therefore, not apply to the whole HFpEF population.
Conclusion
Both CPO and CPORAP are associated with adverse outcomes in patients with HFpEF. By incorporating RAP, CPORAP integrates both cardiac performance and right heart function and could better reflect the true cardiac pump ability in HFpEF. Compared with CPO, CPORAP could enhance the prognostic value. Our data may provide new insights into the assessment of patients with HFpEF, especially those with suspected right heart involvement.
Data Availability
The data will be shared on reasonable request to the corresponding author.
Abbreviations
- AUC:
-
Area under the curve
- BMI:
-
Body mass index
- CO:
-
Cardiac output
- CPO:
-
Cardiac power output
- CPORAP :
-
Right atrial pressure-corrected cardiac power output
- DAP:
-
Diastolic arterial pressure
- dPAP:
-
Diastolic pulmonary arterial pressure
- HF:
-
Heart failure
- HFpEF:
-
Heart failure with preserved ejection fraction
- HFrEF:
-
Heart failure with reduced ejection fraction
- LV-Ea:
-
Left ventricular effective arterial elastance
- LVEF:
-
Left ventricular ejection fraction
- MAP:
-
Mean arterial pressure
- mPAP:
-
Mean pulmonary arterial pressure
- NT-proBNP:
-
N-terminal pro-B-type natriuretic peptide
- NYHA:
-
New York Heart Association
- PAWP:
-
Pulmonary arterial wedge pressure
- PAC:
-
Pulmonary arterial compliance
- PH:
-
Pulmonary hypertension
- PVR:
-
Pulmonary vascular resistance
- RAP:
-
Right atrial pressure
- ROC:
-
Receiver operating characteristic
- RHC:
-
Right heart catheterization
- SAP:
-
Systolic arterial pressure
- sPAP:
-
Systolic pulmonary arterial pressure
- SV:
-
Stroke volume
- SVR:
-
Systemic vascular resistance
- TAPSE:
-
Tricuspid annular plane systolic excursion
References
Fincke R, Hochman JS, Lowe AM, et al. Cardiac power is the strongest hemodynamic correlate of mortality in cardiogenic shock: a report from the SHOCK trial registry. J Am Coll Cardiol. 2004;44(2):340–8. https://doi.org/10.1016/j.jacc.2004.03.060.
Grodin JL, Mullens W, Dupont M, et al. Prognostic role of cardiac power index in ambulatory patients with advanced heart failure. Eur J Heart Fail. 2015;17(7):689–96. https://doi.org/10.1002/ejhf.268.
Yildiz O, Aslan G, Demirozu ZT, Yenigun CD, Yazicioglu N. Evaluation of resting cardiac power output as a prognostic factor in patients with advanced heart failure. Am J Cardiol. 2017;120(6):973–9. https://doi.org/10.1016/j.amjcard.2017.06.028.
Harada T, Yamaguchi M, Omote K, et al. Cardiac power output is independently and incrementally associated with adverse outcomes in heart failure with preserved ejection fraction. Circ Cardiovasc Imaging. 2022;15(2):e013495. https://doi.org/10.1161/CIRCIMAGING.121.013495.
Tan LB. Cardiac pumping capability and prognosis in heart failure. Lancet. 1986;2(8520):1360–3. https://doi.org/10.1016/s0140-6736(86)92006-4.
Lim HS. Cardiac power output revisited. Circ Heart Fail. 2020;13(10):e007393. https://doi.org/10.1161/CIRCHEARTFAILURE.120.007393.
Baldetti L, Pagnesi M, Gallone G, et al. Prognostic value of right atrial pressure-corrected cardiac power index in cardiogenic shock. ESC Heart Fail. 2022;9(6):3920–30. https://doi.org/10.1002/ehf2.14093.
Belkin MN, Alenghat FJ, Besser SA, Pinney SP, Grinstein J. Improved prognostic performance of cardiac power output with right atrial pressure: a subanalysis of the ESCAPE trial. J Card Fail. 2022;28(5):866–9. https://doi.org/10.1016/j.cardfail.2021.11.001.
Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension [published correction appears in Eur Heart J. 2023;44(15):1312. Eur Heart J. 2022;43(38):3618–3731. https://doi.org/10.1093/eurheartj/ehac237
Obokata M, Reddy YNV, Melenovsky V, Pislaru S, Borlaug BA. Deterioration in right ventricular structure and function over time in patients with heart failure and preserved ejection fraction. Eur Heart J. 2019;40(8):689–97. https://doi.org/10.1093/eurheartj/ehy809.
Gorter TM, Obokata M, Reddy YNV, Melenovsky V, Borlaug BA. Exercise unmasks distinct pathophysiologic features in heart failure with preserved ejection fraction and pulmonary vascular disease. Eur Heart J. 2018;39(30):2825–35. https://doi.org/10.1093/eurheartj/ehy331.
Shah SJ, Borlaug BA, Kitzman DW, et al. Research priorities for heart failure with preserved ejection fraction: National Heart, Lung, and Blood Institute Working Group Summary. Circulation. 2020;141(12):1001–26. https://doi.org/10.1161/CIRCULATIONAHA.119.041886.
Inampudi C, Silverman D, Simon MA, et al. Pulmonary hypertension in the context of heart failure with preserved ejection fraction. Chest. 2021;160(6):2232–46. https://doi.org/10.1016/j.chest.2021.08.039.
Borlaug BA, Obokata M. Is it time to recognize a new phenotype? Heart failure with preserved ejection fraction with pulmonary vascular disease. Eur Heart J. 2017;38(38):2874–8. https://doi.org/10.1093/eurheartj/ehx184.
Borlaug BA, Obokata M. The other atrium in heart failure. JACC Cardiovasc Imaging. 2019;12(8 Pt 1):1471–3. https://doi.org/10.1016/j.jcmg.2018.08.019.
Jain S, Kuriakose D, Edelstein I, et al. Right atrial phasic function in heart failure with preserved and reduced ejection graction. JACC Cardiovasc Imaging. 2019;12(8 Pt 1):1460–70. https://doi.org/10.1016/j.jcmg.2018.08.020.
Nagata R, Harada T, Omote K, et al. Right atrial pressure represents cumulative cardiac burden in heart failure with preserved ejection fraction. ESC Heart Fail. 2022;9(2):1454–62. https://doi.org/10.1002/ehf2.13853.
Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J. 2014;35(48):3452–62. https://doi.org/10.1093/eurheartj/ehu193.
Gorter TM, van Veldhuisen DJ, Bauersachs J, et al. Right heart dysfunction and failure in heart failure with preserved ejection fraction: mechanisms and management. Position statement on behalf of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20(1):16–37. https://doi.org/10.1002/ejhf.1029.
Belkin MN, Kalantari S, Kanelidis AJ, et al. Aortic pulsatility index: a novel hemodynamic variable for evaluation of decompensated heart failure. J Card Fail. 2021;27(10):1045–52. https://doi.org/10.1016/j.cardfail.2021.05.010.
Cooper LB, Mentz RJ, Stevens SR, et al. Hemodynamic predictors of heart failure morbidity and mortality: fluid or flow? J Card Fail. 2016;22(3):182–9. https://doi.org/10.1016/j.cardfail.2015.11.012.
Guazzi M, Naeije R. Right heart phenotype in heart failure with preserved ejection fraction. Circ Heart Fail. 2021;14(4):e007840. https://doi.org/10.1161/CIRCHEARTFAILURE.120.007840.
Funding
This work was supported by National Natural Science Foundation of China (grant number 81873472); the Key Projects in the National Science and Technology Pillar Program of the 13th Five-Year Plan Period (grant number 2017YFC1308300), Beijing, People’s Republic of China; the Key Projects in the National Science and Technology Pillar Program of the 12th Five-Year Plan Period (grant number 2011BAI11B08), Beijing, People’s Republic of China; and CAMS Innovation Fund for Medical Science (grant number 2020-I2M-1-002).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study. This study was approved by the Institutional Review Board of Fuwai Hospital (Approval No. 2018-1041).
Conflict of Interest
The authors declare no competing interests.
Additional information
Associate Editor Marat Fudim oversaw the review of this article
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, Y., Tian, P., Liang, L. et al. Improved Prognostic Performance of Right Atrial Pressure-Corrected Cardiac Power Output in Pulmonary Hypertension and Heart Failure with Preserved Ejection Fraction. J. of Cardiovasc. Trans. Res. 17, 448–457 (2024). https://doi.org/10.1007/s12265-023-10429-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-023-10429-y