Abstract
Background
Recent data suggest different causes of renal dysfunction between heart failure with reduced (HFrEF) versus preserved ejection fraction (HFpEF). We therefore studied a wide range of urinary markers reflecting different nephron segments in heart failure patients.
Methods
In 2070, in chronic heart failure patients, we measured several established and upcoming urinary markers reflecting different nephron segments.
Results
Mean age was 70 ± 12 years, 74% was male and 81% (n = 1677) had HFrEF. Mean estimated glomerular filtration rate (eGFR) was lower in patients with HFpEF (56 ± 23 versus 63 ± 23 ml/min/1.73 m2, P = 0.001). Patients with HFpEF had significantly higher values of NGAL (58.1 [24.0–124.8] versus 28.1 [14.6–66.9] μg/gCr, P < 0.001) and KIM-1 (2.28 [1.49–4.37] versus 1.79 [0.85–3.49] μg/gCr, P = 0.001). These differences were more pronounced in patients with an eGFR > 60 ml/min/1.73m2.
Conclusions
HFpEF patients showed more evidence of tubular damage and/or dysfunction compared with HFrEF patients, in particular when glomerular function was preserved.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renal dysfunction is frequently present in patients with heart failure (HF) and is associated with a worse prognosis [1, 2]. This is true for both patients with heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF) [3, 4].
However, since both HFrEF and HFpEF are different disease entities with different pathophysiology and treatment responses, the question remains whether underlying causes for renal dysfunction also differ among the heart failure entities [5, 6]. In a previous study, we showed that an increased urinary albumin excretion and higher cystatin C levels were associated with the risk for the development of HFpEF, but not for HFrEF [7]. A potential explanation of this difference is that renal dysfunction in patients with HFrEF seems to be predominantly related to renal hemodynamic changes, while renal dysfunction in HFpEF seems to be related to endothelial dysfunction and inflammation [8,9,10]. We therefore postulate different drivers for renal dysfunction between patients with HFpEF and HFrEF [9]. To further explore differences in renal pathophysiology between patients with HFrEF and HFpEF, we measured 10 established and emerging urinary markers reflecting different segments of the nephron.
Methods
Study Population
For the current study, we used 2516 patients from the index cohort of BIOSTAT-CHF (A systems BIOlogy Study to Tailored Treatment in Chronic Heart Failure). BIOSTAT-CHF is a multicentre, prospective observational study in two independent cohorts of patients with HF treated with loop diuretics [9, 11,12,13]. The complete list of inclusion and exclusion criteria, and the main outcome of the study, was previously published elsewhere [13,14,15]. The study complied with the Declaration of Helsinki, local ethics committee has approved the research protocol, and all patients signed informed consent. To better establish and distinguish the difference between HFrEF (Left ventricular ejection fraction (LVEF) below 40%) and HFpEF (LVEF equal or above 50%), patients with heart failure with mid-range ejection fraction (LVEF between 40 and 50%) were excluded from the present analysis. Ejection fraction cut-offs were according to the most recent ESC heart failure guidelines [16].
Urinary Analysis
Baseline urine samples and LVEF were available in 2070 patients from the index cohort. Random urine samples were taken at baseline and stored at − 80 °C, and additional methods for the urinary measurements are depicted in Supplementary material. The biomarkers were specifically measured since they are associated with a specific nephron segment via literature research, and therefore could reflect specific injury and/or functional impairment in that part of the nephron (Fig. 1). When available, normal values for urine markers were based on previous research [17, 18]. Urinary albumin and urinary creatinine were considered representative for the glomerulus, urinary neutrophil gelatinase-associated lipocalin (NGAL) and urinary kidney injury molecule-1 (KIM-1) for the proximal tubule, urinary uromodulin for the loop of Henle and urinary osteopontin for the collecting duct [19,20,21,22,23,24,25].
Fractional sodium excretion was calculated by (serum creatinine × urinary sodium)/(serum sodium × urinary creatinine) × 100%. As fractional sodium excretion is more affected by diuretic therapy, we also calculated fractional urea excretion. This was calculated as follows: (serum creatinine × urinary urea)/(serum urea × urinary creatinine) × 100% [26]. By assessing fractional sodium and urea excretion a possible cause for kidney injury can be assessed, i.e. prerenal or intrinsic renal. A fractional sodium excretion below 1% suggests a prerenal cause of the kidney injury, whereas a value of 1% or higher is associated with an intrinsic renal cause for the kidney injury. Fractional urea excretion equal or below 35% was considered prerenal, while 50% or higher was considered to be an intrinsic renal cause. A value between 35 and 50% was found to be indeterminate, and not suggestive for a prerenal or intrinsic renal cause [26]. Microalbuminuria was defined as a urinary albumin/creatinine ratio (UACR) between 2.5 and 25 mg/mmol for men and 3.5 and 35 mg/mmol for women. Macro-albuminuria was defined as a UACR above 25 mg/mmol for men and 35 mg/mmol for women, and a UACR below 2.5 mg/mmol for men and 3.5 mg/mmol for women was considered normal.
Statistical Analysis
Normally distributed data are presented as means and standard deviation, not normally distributed data as medians and 25th until 75th percentile and categorical variables as percentages and frequencies. Intergroup differences were tested using one-way ANOVA for normal distributed data, whereas skewed data was analyzed using the Chi-squared test or Mann–Whitney test depending on whether the data was continuous or nominal.
All non-normally distributed markers were transformed accordingly to the best fit. To assess the association between the different urinary markers and glomerular filtration rate, linear regression was performed in both HFrEF and HFpEF patients, and a P-value for interaction was tested. Associations of the different urinary markers were tested using Cox-proportional hazard models. The multivariable model was corrected for the previously published BIOSTAT risk prediction model [15].
To compare the different nephron segments in HFpEF versus HFrEF, values were standardized. A two-sided P-value < 0.05 was considered statistically significant.
All analyses were performed using IBM SPSS Statistics version 23 and R: a Language and Environment for Statistical Computing, version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline Characteristics
Urinary measurements were available in 2070 patients. Baseline characteristics of these patients are depicted in Table 1. Mean age was 70 ± 12 years, and 74% was male; mean LVEF was 31 ± 11%, and mean eGFR was 61 ± 23 ml/min/1.73 m2.
For the present analyses, we included 1677 patients with HFrEF and 128 patients with HFpEF. Patients with HFmrEF (n = 265) were excluded; however, baseline characteristics including HFmrEF patients are depicted in Supplementary Table 1 and show that these patients are in between HFpEF and HFrEF.
Patients with HFrEF were younger, more often male and had a lower systolic blood pressure but a higher diastolic blood pressure (all P < 0.001) and had a higher eGFR (63 ± 23 versus 56 ± 23 ml/min/1.73 m2, P = 0.001), but serum creatinine levels did not differ (P = 0.513). In patients with HFrEF, 48% had an eGFR < 60 ml/min/1.73 m2, compared with 61% in patients with HFpEF (P = 0.005). Patients with HFrEF more often had a history of myocardial infarction (P < 0.001) and a percutaneous coronary intervention (PCI) (P = 0.030). Patients with HFpEF were more likely to have a history of hypertension and atrial fibrillation (both P < 0.001).
Urinary Markers
Urinary markers are depicted in Table 2. The median UACR in the total cohort was 23.6 [7.29–100.9] mg/gCr, where 770 (37%) of the patients had micro-albuminuria and 265 (13%) macro-albuminuria. The median urinary sodium level was 112.3 [53.0–237.6] mmol/gCr and the median urinary potassium level was 52.9 [36.6–78.9] mmol/gCr.
The median levels of urinary KIM-1 and of urinary NGAL were 1.86 [0.88–3.52] μg/gCr and 30.8 [15.2–74.0], respectively, which were both increased compared to normal values (cut-off value for KIM-1 is 0.98 μg/gCr and for NGAL above 31 μg/gCr) [27].
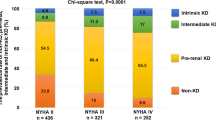
Furthermore, the majority of patients (86%) showed evidence of a prerenal cause for renal dysfunction based on the fraction urea excretion.
Data from patients with HFmrEF showed that these patients’ values were in between the other two heart failure groups (Supplementary Table 2).
Urinary Markers in HFrEF Versus HFpEF
Table 2 shows that patients with HFpEF had significantly higher levels of UACR (P = 0.001), urinary potassium (P = 0.018) and urinary sodium excretion (P = 0.001). In addition, patients with HFpEF had higher levels of the proximal tubular damage markers urinary KIM-1 and urinary NGAL than patients with HFrEF (P = 0.001 and P < 0.001 respectively). Furthermore, HFpEF patients showed significantly higher levels of urinary osteopontin (P = 0.009). Patients with HFpEF had a higher fractional sodium and urea excretion and significantly more intrinsic cause of their renal dysfunction (13% versus 21%, P = 0.036).
In Fig. 2, the standardized levels of the different markers are depicted per nephron segment. When combining the mean standardized values for the different nephron segments, we found significantly higher levels in almost all segments for HFpEF patients, except in the loop of Henle (Fig. 3). To further assess the urinary markers along the eGFR spectrum, patients were divided into eGFR groups. Amongst patients with an eGFR < 45 ml/min/1.73 m2, the only significant difference was found in the proximal tubule, where higher levels were found in patients with HFpEF (Fig. 3). To assess the associates of eGFR, univariable linear regression was performed in the two subgroups (Table 3). In patients with HFrEF, lower levels of KIM-1 and NGAL were significantly associated with a higher eGFR (both P < 0.001), while for uromodulin, higher levels were significantly associated with a higher eGFR (P = 0.005). In patients with HFpEF, only uromodulin was significantly associated with eGFR (P = 0.001), with a significant interaction between the heart failure subgroups (P = 0.013).
Since eGFR was slightly different between the groups, markers were stratified in different eGFR groups and shown in Table 4. In patients with an eGFR < 45 ml/min/1.73m2, urinary NGAL levels and UACR were higher in HFpEF patients (P = 0.017 and P = 0.009 respectively), while in patients with an eGFR between 45 and 60 ml/min/1.73 m2, no significant differences were found. However, in HF patients with a normal renal function (eGFR > 60 ml/min/1.73m2), we found significantly higher levels for almost all urinary markers in HFpEF patients compared with patients with HFrEF: urinary KIM-1 (P = 0.049), urinary NGAL (P < 0.001), urinary osteopontin (P = 0.001), urinary uromodulin (P = 0.044) and UACR (P = 0.007), while urinary creatinine levels were significantly lower in HFpEF patients (P = 0.003).
Lastly, the association between the urinary markers and all-cause mortality is assessed and depicted in Supplementary Table 3. In a univariable model KIM-1, NGAL and osteopontin were significantly associated with all-cause mortality; however, in a multivariable model corrected for the previously published risk prediction model, none of the markers were significantly associated with mortality.
Discussion
In a large cohort of chronic HF patients with a high prevalence of renal glomerular dysfunction, we found marked differences between patients with HFrEF and HFpEF. In patients with HFpEF, more (proximal) tubular damage/dysfunction was observed than in patients with HFrEF. This difference in renal tubular pathophysiology between patients with HFrEF and HFpEF was most pronounced in patients with preserved glomerular function.
Renal Function and Heart Failure
Although renal dysfunction in HF has been studied for several years, the majority of the studies focused on glomerular function, although renal function is much more than GFR alone [28]. Urinary measurements could provide more insight in the pathophysiological mechanism behind renal dysfunction in patients with HF. One of the urinary markers often studied is albuminuria. We found microalbuminuria in 37% of the HF patients and macroalbuminuria in 13% of the patients, which is consistent with previous studies [29]. However, other urinary markers in HF populations are often single-biomarker measurements studied to a limited extent, or not even measured at all. This is the first study to assess several standard urinary measurements and urinary markers associated with different nephron segments in a large HF cohort.
Renal Dysfunction in Heart Failure with Preserved and Reduced Ejection Fraction
Cardiorenal interaction has been mainly studied in patients with HFrEF. However, the prevalence of renal impairment is similar in patients with HFpEF and associated with increased mortality risks in both groups [30]. Nevertheless, factors underlying renal dysfunction might be different between patients with HFpEF versus patients with HFrEF.
Haemodynamics play an important role in the pathophysiology of renal dysfunction in patients with HF. A reduced renal blood flow and increased central venous pressure have been known as proven contributors in renal dysfunction [28, 31, 32]. In this study, we showed that the majority of patients had a prerenal cause of renal dysfunction, yet for patients with HFpEF, there was a significantly higher incidence of intrinsic renal dysfunction. As a prerenal factor, decreased renal blood flow due to forward failure is more likely to play a role in renal dysfunction in HFrEF patients. The higher incidence of intrinsic renal dysfunction in HFpEF might be due to the association of chronic kidney disease and HFpEF with endothelial dysfunction and inflammation. The microvascular changes present in both are likely to play a role in the progression of both the HFpEF and renal dysfunction. Another possible explanation for the microvascular dysfunction could be oxidative stress, caused by toxins increasing reactive oxygen species [33]. Moreover, studies link oxidative stress as an important factor in HFpEF, leading to a chronic state of low-grade inflammation, and with that enhancing the endothelial dysfunction in these patients [9, 34, 35].
Additionally, we measured several urinary markers linked to different nephron segments and analysed these markers over the entire renal continuum. We found that established markers for tubular dysfunction and injury were elevated compared with healthy subjects in patients with HFrEF and HFpEF. However, tubular dysfunction was more pronounced in patients with HFpEF. With decreasing eGFR, we found that levels of both markers of tubular dysfunction, urinary KIM-1 and urinary NGAL, increased with decreasing eGFR [36, 37]. Interestingly, the difference in tubular markers between patients with HFrEF and HFpEF was particularly present in patients with a preserved eGFR. This might imply that in patients with HFpEF, renal dysfunction is already present, even when glomerular function is still preserved. Proximal tubular damage is a modulating factor in the progression of CKD, and due to its high oxygen consumption, the tubule is particularly vulnerable to damage [38]. Since eGFR merely estimates the filtration capacity of the kidney, solely relying on this marker could underappreciate possible underlying damage downstream of Bowman’s capsule, especially in HFpEF. Proximal tubular damage is not only linked to progression of CKD, but also activates various inflammatory cytokines due to damage to the proximal tubular cells in early states preceding damage [39]. Overall, our data show that proximal tubule damage is most abundant in patients with HFpEF with a preserved renal function, and we found that in patients with HFpEF, the injury seems to be more throughout the entire nephron.
Study Limitations
Firstly, we used spot urine samples obtained at random time points since 24-h urine samples were not available in this cohort. Secondly, the number of HFpEF patients is limited in our cohort with a high percentage of male patients in the cohort. Thirdly, we only have a single measurement available, so conclusions about the course of renal dysfunction cannot be drawn. Lastly, based on previous studies, we have linked certain urinary markers specifically to one nephron segment; however, an interaction with another nephron segment cannot be ruled out. Furthermore, due to the cross-sectional nature of this study, causality cannot be proven, and these data should be considered hypothesis generating.
Conclusion
In patients with a preserved glomerular function, proximal tubular dysfunction is more prevalent in patients with HFpEF compared with patients with HFrEF, suggesting different underlying renal pathophysiology between patients with HFpEF and HFrEF.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
Abbreviations
- HFrEF:
-
Heart failure with reduced ejection fraction
- HFpEF:
-
Heart failure with preserved ejection fraction
- eGFR:
-
Estimated glomerular filtration rate
- HF:
-
Heart failure
- BIOSTAT-CHFA systems:
-
BIOlogy Study to Tailored Treatment in Chronic Heart Failure
- LVEF:
-
Left ventricular ejection fraction
- NGAL:
-
Neutrophil gelatinase-associated lipocalin
- KIM-1:
-
Kidney injury molecule-1
- UACR:
-
Urinary albumin/creatinine ratio
References
Damman K, Voors AA, Navis G, van Veldhuisen DJ, Hillege HL. The cardiorenal syndrome in heart failure. Prog Cardiovasc Dis. 2011;54:144–53.
Hillege HL, Girbes AR, de Kam PJ, Boomsma F, de Zeeuw D, Charlesworth A, Hampton JR, van Veldhuisen DJ. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation. 2000;102:203–10.
Damman K, Valente MA, Voors AA, O’Connor CM, van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J. 2014;35:455–69.
Streng KW, Nauta JF, Hillege HL, Anker SD, Cleland JG, Dickstein K, Filippatos G, Lang CC, Metra M, Ng LL, Ponikowski P, Samani NJ, van Veldhuisen DJ, Zwinderman AH, Zannad F, Damman K, van dM, Voors AA. Non-cardiac comorbidities in heart failure with reduced, mid-range and preserved ejection fraction. Int J Cardiol. 2018.
Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2014;11:507–15.
Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670–9.
Brouwers FP, de Boer RA, van der Harst P, Voors AA, Gansevoort RT, Bakker SJ, Hillege HL, van Veldhuisen DJ, van Gilst WH. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur Heart J. 2013;34:1424–31.
McAlister FA, Ezekowitz J, Tarantini L, Squire I, Komajda M, Bayes-Genis A, Gotsman I, Whalley G, Earle N, Poppe KK, Doughty RN. Meta-analysis Global Group in Chronic Heart Failure (MAGGIC) investigators. Renal dysfunction in patients with heart failure with preserved versus reduced ejection fraction: impact of the new Chronic Kidney Disease-Epidemiology Collaboration Group formula. Circ Heart Fail. 2012;5:309–14.
Ter Maaten JM, Damman K, Verhaar MC, Paulus WJ, Duncker DJ, Cheng C, van Heerebeek L, Hillege HL, Lam CS, Navis G, Voors AA. Connecting heart failure with preserved ejection fraction and renal dysfunction: the role of endothelial dysfunction and inflammation. Eur J Heart Fail. 2016;18:588–98.
Schroten NF, Damman K, Valente MA, Smilde TD, van Veldhuisen DJ, Navis G, Gaillard CA, Voors AA, Hillege HL. Long-term changes in renal function and perfusion in heart failure patients with reduced ejection fraction. Clin Res Cardiol. 2016;105:10–6.
Ferreira JP, Rossignol P, Machu JL, Sharma A, Girerd N, Anker SD, Cleland JG, Dickstein K, Filippatos G, Hillege HL, Lang CC, Ter Maaten JM, Metra M, Ng L, Ponikowski P, Samani NJ, van Veldhuisen DJ, Zwinderman AH, Voors A, Zannad F. Mineralocorticoid receptor antagonist pattern of use in heart failure with reduced ejection fraction: findings from BIOSTAT-CHF. Eur J Heart Fail. 2017;19:1284–93.
Ouwerkerk W, Zwinderman AH, Ng LL, Demissei B, Hillege HL, Zannad F, van Veldhuisen DJ, Samani NJ, Ponikowski P, Metra M, Ter Maaten JM, Lang CC, van der Harst P, Filippatos G, Dickstein K, Cleland JG, Anker SD, Voors AA. Biomarker-guided versus guideline-based treatment of patients with heart failure: results From BIOSTAT-CHF. J Am Coll Cardiol. 2018;71:386–98.
Voors AA, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P, Hillege HL, Lang CC, Ter Maaten JM, Ng L, Ponikowski P, Samani NJ, van Veldhuisen DJ, Zannad F, Zwinderman AH, Metra M. A systems BIOlogy Study to TAilored Treatment in Chronic Heart Failure: rationale, design, and baseline characteristics of BIOSTAT-CHF. Eur J Heart Fail. 2016;18:716–26.
Ouwerkerk W, Voors AA, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P, Hillege HL, Lang CC, Ter Maaten JM, Ng LL, Ponikowski P, Samani NJ, van Veldhuisen DJ, Zannad F, Metra M, Zwinderman AH. Determinants and clinical outcome of uptitration of ACE-inhibitors and beta-blockers in patients with heart failure: a prospective European study. Eur Heart J. 2017;38:1883–90.
Voors AA, Ouwerkerk W, Zannad F, van Veldhuisen DJ, Samani NJ, Ponikowski P, Ng LL, Metra M, Ter Maaten JM, Lang CC, Hillege HL, van der Harst P, Filippatos G, Dickstein K, Cleland JG, Anker SD, Zwinderman AH. Development and validation of multivariable models to predict mortality and hospitalization in patients with heart failure. Eur J Heart Fail. 2017;19:627–34.
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force Members. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–200.
Chaturvedi S, Farmer T, Kapke GF. Assay validation for KIM-1: human urinary renal dysfunction biomarker. Int J Biol Sci. 2009;5:128–34.
Cullen MR, Murray PT, Fitzgibbon MC. Establishment of a reference interval for urinary neutrophil gelatinase-associated lipocalin. Ann Clin Biochem. 2012;49:190–3.
Currie G, McKay G, Delles C. Biomarkers in diabetic nephropathy: present and future. World J Diabetes. 2014;5:763–76.
Curthoys NP, Moe OW. Proximal tubule function and response to acidosis. Clin J Am Soc Nephrol. 2014;9:1627–38.
Kamijo A, Kimura K, Sugaya T, Yamanouchi M, Hikawa A, Hirano N, Hirata Y, Goto A, Omata M. Urinary fatty acid-binding protein as a new clinical marker of the progression of chronic renal disease. J Lab Clin Med. 2004;143:23–30.
Lameire N, Van Biesen W, Vanholder R. Acute kidney injury. Lancet. 2008;372:1863–5.
Nijst P, Verbrugge FH, Grieten L, Dupont M, Steels P, Tang WH, Mullens W. The pathophysiological role of interstitial sodium in heart failure. J Am Coll Cardiol. 2015;65:378–88.
Ohara N, Hanyu O, Hirayama S, Nakagawa O, Aizawa Y, Ito S, Sone H. Hypertension increases urinary excretion of immunoglobulin G, ceruloplasmin and transferrin in normoalbuminuric patients with type 2 diabetes mellitus. J Hypertens. 2014;32:432–8.
Pruijm M, Ponte B, Ackermann D, Paccaud F, Guessous I, Ehret G, Pechere-Bertschi A, Vogt B, Mohaupt MG, Martin PY, Youhanna SC, Nagele N, Vollenweider P, Waeber G, Burnier M, Devuyst O, Bochud M. Associations of urinary uromodulin with clinical characteristics and markers of tubular function in the general population. Clin J Am Soc Nephrol. 2016;11:70–80.
Carvounis CP, Nisar S, Guro-Razuman S. Significance of the fractional excretion of urea in the differential diagnosis of acute renal failure. Kidney Int. 2002;62:2223–9.
Schinstock CA, Semret MH, Wagner SJ, Borland TM, Bryant SC, Kashani KB, Larson TS, Lieske JC. Urinalysis is more specific and urinary neutrophil gelatinase-associated lipocalin is more sensitive for early detection of acute kidney injury. Nephrol Dial Transplant. 2013;28:1175–85.
Damman K, Testani JM. The kidney in heart failure: an update. Eur Heart J. 2015;36:1437–44.
Jackson CE, Solomon SD, Gerstein HC, Zetterstrand S, Olofsson B, Michelson EL, Granger CB, Swedberg K, Pfeffer MA, Yusuf S, McMurray JJ. CHARM Investigators and Committees. Albuminuria in chronic heart failure: prevalence and prognostic importance. Lancet. 2009;374:543–50.
Ather S, Chan W, Bozkurt B, Aguilar D, Ramasubbu K, Zachariah AA, Wehrens XH, Deswal A. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol. 2012;59:998–1005.
Nijst P, Martens P, Dupont M, Tang WHW, Mullens W. Intrarenal flow alterations during transition from euvolemia to intravascular volume expansion in heart failure patients. JACC Heart Fail. 2017;5:672–81.
Damman K, Navis G, Smilde TD, Voors AA, van der Bij W, van Veldhuisen DJ, Hillege HL. Decreased cardiac output, venous congestion and the association with renal impairment in patients with cardiac dysfunction. Eur J Heart Fail. 2007;9:872–8.
van der Pol A, Gil A, Sillje HHW, Tromp J, Ovchinnikova ES, Vreeswijk-Baudoin I, Hoes M, Domian IJ, van de Sluis B, van Deursen JM, Voors AA, van Veldhuisen DJ, van Gilst WH, Berezikov E, van der Harst P, de Boer RA, Bischoff R, van der Meer P. Accumulation of 5-oxoproline in myocardial dysfunction and the protective effects of OPLAH. Sci Transl Med. 2017; 9: https://doi.org/10.1126/scitranslmed.aam8574.
Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–71.
Fang JC. Heart failure with preserved ejection fraction: a kidney disorder? Circulation. 2016;134:435–7.
Torregrosa I, Montoliu C, Urios A, Andres-Costa MJ, Gimenez-Garzo C, Juan I, Puchades MJ, Blasco ML, Carratala A, Sanjuan R, Miguel A. Urinary KIM-1, NGAL and L-FABP for the diagnosis of AKI in patients with acute coronary syndrome or heart failure undergoing coronary angiography. Heart Vessels. 2014; .
van Veldhuisen DJ, Ruilope LM, Maisel AS, Damman K. Biomarkers of renal injury and function: diagnostic, prognostic and therapeutic implications in heart failure. Eur Heart J. 2016;37:2577–85.
Chevalier RL. The proximal tubule is the primary target of injury and progression of kidney disease: role of the glomerulotubular junction. Am J Physiol Renal Physiol. 2016;311:F145–61.
Nakhoul N, Batuman V. Role of proximal tubules in the pathogenesis of kidney disease. Contrib Nephrol. 2011;169:37–50.
Funding
This work was supported by the Netherlands Cardiovascular Research Initiative: an initiative with support of the Dutch Heart Foundation [CVON2014-11 RECONNECT] and a grant from the European Commission [FP7-242209-BIOSTAT-CHF]. There is no relation with industry.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
The study complied with the Declaration of Helsinki, the local ethics committee has approved the research protocol, and all the patients signed informed consent.
Competing Interests
M.M. received the following personal fees of minimal amounts in the last 3 years: from Actelion, Amgen, Livanova, Servier and Vifor pharma as member of Executive or Data Monitoring Committees of sponsored clinical trials; from Astra-Zeneca, Abbott vascular, Bayer, Boheringer Ingelhelm and Edwards Therapeutics for participation to advisory boards and/or speeches at sponsored meetings. S.D.A. reports receiving fees from Abbott, Actimed, Bayer, Boehringer Ingelheim, Cardiac Dimension, Cordio, Impulse Dynamics, Novartis, Occlutech, Servier and Vifor Pharma and grant support from Abbott and Vifor Pharma. C.C.L. received consultancy fees and/or research grants from Amgen, Astra Zeneca, MSD, Novartis and Servier. A.A.V Amgen, Bayer, Boehringer Ingelheim, Merck/MSD, Novartis, Roche Diagnostics, Servier, Trevena and Vifor. All the other authors declare no conflict of interest.
Additional information
Associate Editor Craig M. Stolen oversaw the review of this article
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Streng, K.W., Hillege, H.L., ter Maaten, J.M. et al. Urinary Marker Profiles in Heart Failure with Reduced Versus Preserved Ejection Fraction. J. of Cardiovasc. Trans. Res. 17, 3–12 (2024). https://doi.org/10.1007/s12265-023-10356-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-023-10356-y