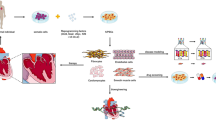
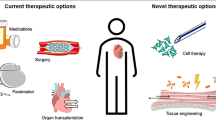
Abstract
Stem cell-based therapy for ischemic heart disease (IHD) has become a promising but controversial strategy during the past two decades. The fate and effects of stem cells engrafted into ischemia myocardium are still not fully understood. Stem cell-derived exosomes, a subcategory of extracellular vesicles with nano size, have been considered as an efficient and safe transporter for microRNAs (miRNAs) and a central mediator of the cardioprotective potentials of the parental cells. Hypoxia, pharmacological intervention, and gene manipulation could alter the exosomal miRNAs cargos from stem cells and promote therapeutic potential. Furthermore, several bioengineering methods were also successfully applied to modify miRNAs content and components of exosomal membrane proteins recently. In this review, we outline relevant results about exosomal miRNAs from stem cells and focus on the current strategies to promote their therapeutic efficiency in IHD.

Similar content being viewed by others
Abbreviations
- BMSC:
-
Bone marrow mesenchymal stem cells
- CCND2:
-
Cyclin D2
- CDC:
-
Cardiosphere-derived cells
- Cltc1:
-
Clathrin heavy chain 1
- CNP:
-
Cellular nanoporation biological chip
- COX-2:
-
Cyclooxygenase 2
- CPC:
-
Cardiac progenitor cells
- ERCC:
-
Extracellular RNA Communication Consortium
- ESC:
-
Embryonic stem cells
- hucMSC:
-
Human umbilical cord MSCs
- HUVEC:
-
Human umbilical vein endothelial cell
- IHD:
-
Ischemic heart disease
- iPSC:
-
Induced pluripotent cell
- Lamp2b:
-
Lysosomal-associated membrane protein 2
- MAPC:
-
Multipotent adult progenitor cells
- MI:
-
Myocardial infarction
- MI/R:
-
Myocardial ischemia–reperfusion
- NSC:
-
Neural stem cells
- nSMase2:
-
Neutral sphingomyelinase 2
- PCR:
-
Principal component regression
- PLSR:
-
Partial least squares regression
- PTEN:
-
Gene of phosphate and tension homology deleted on chromsome ten
- PDCD4:
-
Programmed cell death 4
References
Zhao, D., Liu, J., Wang, M., et al. (2019). Epidemiology of cardiovascular disease in China: Current features and implications. Nature Reviews. Cardiology, 16, 203–212. https://doi.org/10.1038/s41569-018-0119-4
Kaski, J.-C., Crea, F., Gersh, B. J., & Camici, P. G. (2018). Reappraisal of ischemic heart disease. Circulation, 138, 1463–1480. https://doi.org/10.1161/CIRCULATIONAHA.118.031373
van der Pol, A., van Gilst, W. H., Voors, A. A., & van der Meer, P. (2019). Treating oxidative stress in heart failure: Past, present and future. European Journal of Heart Failure, 21, 425–435. https://doi.org/10.1002/ejhf.1320
Bai, X., Yan, Y., Song, Y.-H., et al. (2010). Both cultured and freshly isolated adipose tissue-derived stem cells enhance cardiac function after acute myocardial infarction. European Heart Journal, 31, 489–501. https://doi.org/10.1093/eurheartj/ehp568
Wang, J., Chen, Z., Dai, Q., et al. (2020). Intravenously delivered mesenchymal stem cells prevent microvascular obstruction formation after myocardial ischemia/reperfusion injury. Basic Research in Cardiology, 115, 40. https://doi.org/10.1007/s00395-020-0800-8
Gao, L., Gregorich, Z. R., Zhu, W., et al. (2018). Large cardiac muscle patches engineered from human induced-pluripotent stem cell–derived cardiac cells improve recovery from myocardial infarction in swine. Circulation, 137, 1712–1730. https://doi.org/10.1161/CIRCULATIONAHA.117.030785
Shiba, Y., Gomibuchi, T., Seto, T., et al. (2016). Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature, 538, 388–391. https://doi.org/10.1038/nature19815
Alvarez-Dolado, M., Pardal, R., Garcia-Verdugo, J. M., et al. (2003). Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature, 425, 968–973. https://doi.org/10.1038/nature02069
Nygren, J. M., Jovinge, S., Breitbach, M., et al. (2004). Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nature Medicine, 10, 494–501. https://doi.org/10.1038/nm1040
Thum, T., Bauersachs, J., Poole-Wilson, P. A., et al. (2005). The dying stem cell hypothesis. Journal of the American College of Cardiology, 46, 1799–1802. https://doi.org/10.1016/j.jacc.2005.07.053
Vagnozzi, R. J., Maillet, M., Sargent, M. A., et al. (2019). An acute immune response underlies the benefit of cardiac stem-cell therapy. Nature, 577,. https://doi.org/10.1038/s41586-019-1802-2
Gomzikova, M. O., & Rizvanov, A. A. (2017). Current trends in regenerative medicine: From cell to cell-free therapy. Bionanoscience, 7, 240–245. https://doi.org/10.1007/s12668-016-0348-0
Kalluri, R., & LeBleu, V. S. (2020). The biology, function, and biomedical applications of exosomes. Science, 367,. https://doi.org/10.1126/science.aau6977
Moghaddam, A. S., Afshari, J. T., Esmaeili, S. A., et al. (2019). Cardioprotective microRNAs: Lessons from stem cell-derived exosomal microRNAs to treat cardiovascular disease. Atherosclerosis, 285, 1–9. https://doi.org/10.1016/j.atherosclerosis.2019.03.016
Hashimoto, Y., Akiyama, Y., & Yuasa, Y. (2013). Multiple-to-multiple relationships between microRNAs and target genes in gastric cancer. PLoS ONE, 8, e62589. https://doi.org/10.1371/journal.pone.0062589
Agarwal, U., George, A., Bhutani, S., et al. (2017). Experimental, systems, and computational approaches to understanding the microRNA-mediated reparative potential of cardiac progenitor cell–derived exosomes from pediatric patients. Circulation Research, 120, 701–712. https://doi.org/10.1161/CIRCRESAHA.116.309935
Ferguson, S. W., Wang, J., Lee, C. J., et al. (2018). The microRNA regulatory landscape of MSC-derived exosomes: A systems view. Science and Reports, 8, 1–12. https://doi.org/10.1038/s41598-018-19581-x
Das, S., Abdel-Mageed, A. B., Adamidi, C., et al. (2019). The extracellular RNA communication consortium: Establishing foundational knowledge and technologies for extracellular RNA research. Cell, 177, 231–242. https://doi.org/10.1016/j.cell.2019.03.023
Wang, X., Tang, Y., Liu, Z., et al. (2021). The application potential and advance of mesenchymal stem cell-derived exosomes in myocardial infarction. Stem Cells Int, 2021,. https://doi.org/10.1155/2021/5579904
Orlic, D., Kajstura, J., Chimenti, S., et al. (2001). Bone marrow cells regenerate infarcted myocardium. Nature, 410, 701–705. https://doi.org/10.1038/35070587
Tseliou, E., Pollan, S., Malliaras, K., et al. (2013). Allogeneic cardiospheres safely boost cardiac function and attenuate adverse remodeling after myocardial infarction in immunologically mismatched rat strains. Journal of the American College of Cardiology, 61, 1108–1119. https://doi.org/10.1016/j.jacc.2012.10.052
Malliaras, K., Smith, R. R., Kanazawa, H., et al. (2013). Validation of contrast-enhanced magnetic resonance imaging to monitor regenerative efficacy after cell therapy in a porcine model of convalescent myocardial infarction. Circulation, 128, 2764–2775. https://doi.org/10.1161/CIRCULATIONAHA.113.002863
Chimenti, I., Smith, R. R., Li, T.-S., et al. (2010). Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circulation Research, 106, 971–980. https://doi.org/10.1161/CIRCRESAHA.109.210682
Breitbach, M., Bostani, T., Roell, W., et al. (2007). Potential risks of bone marrow cell transplantation into infarcted hearts. Blood, 110, 1362–1369. https://doi.org/10.1182/blood-2006-12-063412
Sun, L., Xu, R., Sun, X., et al. (2016). Safety evaluation of exosomes derived from human umbilical cord mesenchymal stromal cell. Cytotherapy, 18, 413–422. https://doi.org/10.1016/j.jcyt.2015.11.018
Anjos-Afonso, F., Siapati, E. K., & Bonnet, D. (2004). In vivo contribution of murine mesenchymal stem cells into multiple cell-types under minimal damage conditions. Journal of Cell Science, 117, 5655–5664. https://doi.org/10.1242/jcs.01488
Fischer, U. M., Harting, M. T., Jimenez, F., et al. (2009). Pulmonary passage is a major obstacle for intravenous stem cell delivery: The pulmonary first-pass effect. Stem Cells Dev, 18, 683–691. https://doi.org/10.1089/scd.2008.0253
Takahashi, M., Li, T. S., Suzuki, R., et al. (2006). Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. Am J Physiol - Hear Circ Physiol, 291, 886–893. https://doi.org/10.1152/ajpheart.00142.2006
Lee, K., Silva, E. A., & Mooney, D. J. (2011). Growth factor delivery-based tissue engineering: General approaches and a review of recent developments. Journal of the Royal Society, Interface, 8, 153–170. https://doi.org/10.1098/rsif.2010.0223
Eppler, S. M., Combs, D. L., Henry, T. D., et al. (2002). A target-mediated model to describe the pharmacokinetics and hemodynamic effects of recombinant human vascular endothelial growth factor in humans*. Clinical Pharmacology and Therapeutics, 72, 20–32. https://doi.org/10.1067/mcp.2002.126179
Yu, B., Kim, H. W., Gong, M., et al. (2015). Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. International Journal of Cardiology, 182, 349–360. https://doi.org/10.1016/j.ijcard.2014.12.043
Yang, Z., Shi, J., Xie, J., et al. (2020). Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat Biomed Eng, 4, 69–83. https://doi.org/10.1038/s41551-019-0485-1
Arslan, F., Lai, R. C., Smeets, M. B., et al. (2013). Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res, 10, 301–312. https://doi.org/10.1016/j.scr.2013.01.002
Pêche, H., Heslan, M., Usal, C., et al. (2003). Presentation of donor major histocompatibility complex antigens by bone marrow dendritic cell-derived exosomes modulates allograft rejection1. Transplantation, 76, 1503–1510. https://doi.org/10.1097/01.TP.0000092494.75313.38
Lai, R. C., Arslan, F., Lee, M. M., et al. (2010). Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res, 4, 214–222. https://doi.org/10.1016/j.scr.2009.12.003
Yu, B., Gong, M., Wang, Y., et al. (2013). Cardiomyocyte protection by GATA-4 gene engineered mesenchymal stem cells is partially mediated by translocation of miR-221 in microvesicles. PLoS ONE, 8, 1–11. https://doi.org/10.1371/journal.pone.0073304
Wan, Z., Zhao, L., Lu, F., et al. (2020). Mononuclear phagocyte system blockade improves therapeutic exosome delivery to the myocardium. Theranostics, 10, 218–230. https://doi.org/10.7150/thno.38198
Kamerkar, S., LeBleu, V. S., Sugimoto, H., et al. (2017). Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature, 546, 498–503. https://doi.org/10.1038/nature22341
Mayourian, J., Ceholski, D. K., Gorski, P. A., et al. (2018). Exosomal microRNA-21-5p mediates mesenchymal stem cell paracrine effects on human cardiac tissue contractility. Circulation Research, 122, 933–944. https://doi.org/10.1161/CIRCRESAHA.118.312420
Luther, K. M., Haar, L., McGuinness, M., et al. (2018). Exosomal miR-21a-5p mediates cardioprotection by mesenchymal stem cells. Journal of Molecular and Cellular Cardiology, 119, 125–137. https://doi.org/10.1016/j.yjmcc.2018.04.012
Zhao, J., Li, X., Hu, J., et al. (2019). Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovascular Research, 115, 1205–1216. https://doi.org/10.1093/cvr/cvz040
Xiao, C., Wang, K., Xu, Y., et al. (2018). Transplanted mesenchymal stem cells reduce autophagic flux in infarcted hearts via the exosomal transfer of miR-125b. Circulation Research, 123, 564–578. https://doi.org/10.1161/CIRCRESAHA.118.312758
Mohyeldin, A., Garzón-Muvdi, T., & Quiñones-Hinojosa, A. (2010). Oxygen in stem cell biology: A critical component of the stem cell niche. Cell Stem Cell, 7, 150–161. https://doi.org/10.1016/j.stem.2010.07.007
Zhu, L. P., Tian, T., Wang, J. Y., et al. (2018). Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics, 8, 6163–6177. https://doi.org/10.7150/thno.28021
Zhu, J., Lu, K., Zhang, N., et al. (2018). Myocardial reparative functions of exosomes from mesenchymal stem cells are enhanced by hypoxia treatment of the cells via transferring microRNA-210 in an nSMase2-dependent way. Artif Cells, Nanomedicine Biotechnol, 46, 1659–1670. https://doi.org/10.1080/21691401.2017.1388249
Cheng, H., Chang, S., Xu, R., et al. (2020). Hypoxia-challenged MSC-derived exosomes deliver miR-210 to attenuate post-infarction cardiac apoptosis. Stem Cell Research & Therapy, 11, 1–14. https://doi.org/10.1186/s13287-020-01737-0
Zhang, X., Wang, X., Zhu, H., et al. (2010). Synergistic effects of the GATA-4-mediated miR-144/451 cluster in protection against simulated ischemia/reperfusion-induced cardiomyocyte death. Journal of Molecular and Cellular Cardiology, 49, 841–850. https://doi.org/10.1016/j.yjmcc.2010.08.007
Dai, G., Xu, Q., Luo, R., et al. (2015). Atorvastatin treatment improves effects of implanted mesenchymal stem cells: Meta-analysis of animal models with acute myocardial infarction. BMC Cardiovascular Disorders, 15, 1–6. https://doi.org/10.1186/s12872-015-0162-6
Kim, Y. S., Ahn, Y., Kwon, J. S., et al. (2012). Priming of mesenchymal stem cells with oxytocin enhances the cardiac repair in ischemia/reperfusion injury. Cells, Tissues, Organs, 195, 428–442. https://doi.org/10.1159/000329234
Xu, H., Yang, Y. J., Qian, H. Y., et al. (2011). Rosuvastatin treatment activates JAK-STAT pathway and increases efficacy of allogeneic mesenchymal stem cell transplantation in infarcted hearts. Circulation Journal, 75, 1476–1485. https://doi.org/10.1253/circj.CJ-10-1275
Huang, P., Wang, L., Li, Q., et al. (2020). Atorvastatin enhances the therapeutic efficacy of mesenchymal stem cells-derived exosomes in acute myocardial infarction via up-regulating long non-coding RNA H19. Cardiovascular Research, 116, 353–367. https://doi.org/10.1093/cvr/cvz139
Beltrami, A. P., Barlucchi, L., Torella, D., et al. (2003). Adult cardiac stem cells are multipotent and support myocardial regeneration we have documented the existence of cycling ventricular myocytes in the normal and pathologic adult mam. Cell, 114, 763–776.
Chen, L., Wang, Y., Pan, Y., et al. (2013). Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/reperfusion injury. Biochem Biophys Res Commun, 431, 566–571. https://doi.org/10.1016/j.bbrc.2013.01.015
Xiao, J., Pan, Y., Li, X. H., et al. (2016). Cardiac progenitor cell-derived exosomes prevent cardiomyocytes apoptosis through exosomal miR-21 by targeting PDCD4. Cell Death & Disease, 7, 1–10. https://doi.org/10.1038/cddis.2016.181
Gray, W. D., French, K. M., Ghosh-Choudhary, S., et al. (2015). Identification of Therapeutic covariant microRNA clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. Circulation Research, 116, 255–263. https://doi.org/10.1161/CIRCRESAHA.116.304360
Chen, S. J., Chang, C. M., Tsai, S. K., et al. (2010). Functional improvement of focal cerebral ischemia injury by subdural transplantation of induced pluripotent stem cells with fibrin glue. Stem Cells Dev, 19, 1757–1767. https://doi.org/10.1089/scd.2009.0452
Tsuji, O., Miura, K., Okada, Y., et al. (2010). Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proc Natl Acad Sci U S A, 107, 12704–12709. https://doi.org/10.1073/pnas.0910106107
Wang, Y., Zhang, L., Li, Y., et al. (2015). Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. International Journal of Cardiology, 192, 61–69. https://doi.org/10.1016/j.ijcard.2015.05.020
Zhao, M., Nakada, Y., Wei, Y., et al. (2021). Cyclin D2 overexpression enhances the efficacy of human induced pluripotent stem cell-derived cardiomyocytes for myocardial repair in a swine model of myocardial infarction. Circulation, 210–228,. https://doi.org/10.1161/circulationaha.120.049497
Kurtzwald-Josefson, E., Zeevi-Levin, N., Rubchevsky, V., et al. (2020). Cardiac fibroblast-induced pluripotent stem cell-derived exosomes as a potential therapeutic mean for heart failure. International Journal of Molecular Sciences, 21, 1–15. https://doi.org/10.3390/ijms21197215
Lee, W. H., Chen, W.-Y., Shao, N.-Y., et al. (2017). Comparison of non-coding RNAs in exosomes and functional efficacy of human embryonic stem cell- versus induced pluripotent stem cell-derived cardiomyocytes. Stem Cells, 35, 2138–2149. https://doi.org/10.1002/stem.2669
Vaskova, E., Ikeda, G., Tada, Y., et al. (2020). Sacubitril/valsartan improves cardiac function and decreases myocardial fibrosis via downregulation of exosomal mir-181a in a rodent chronic myocardial infarction model. Journal of the American Heart Association, 9,. https://doi.org/10.1161/JAHA.119.015640
Ma, T., Chen, Y., Chen, Y., et al. (2018). MicroRNA-132, delivered by mesenchymal stem cell-derived exosomes, promote angiogenesis in myocardial infarction. Stem Cells Int, 2018,. https://doi.org/10.1155/2018/3290372
Kanki, S., Jaalouk, D. E., Lee, S., et al. (2011). Identification of targeting peptides for ischemic myocardium by in vivo phage display. Journal of Molecular and Cellular Cardiology, 50, 841–848. https://doi.org/10.1016/j.yjmcc.2011.02.003
Wang, X., Chen, Y., Zhao, Z., et al. (2018). Engineered exosomes with ischemic myocardium-targeting peptide for targeted therapy in myocardial infarction. Journal of the American Heart Association, 7, 1–16. https://doi.org/10.1161/JAHA.118.008737
Vandergriff, A., Huang, K., Shen, D., et al. (2018). Targeting regenerative exosomes to myocardial infarction using cardiac homing peptide. Theranostics, 8, 1869–1878. https://doi.org/10.7150/thno.20524
Antes, T. J., Middleton, R. C., Luther, K. M., et al. (2018). Targeting extracellular vesicles to injured tissue using membrane cloaking and surface display. J Nanobiotechnology, 16, 1–15. https://doi.org/10.1186/s12951-018-0388-4
Kim, H., Yun, N., Mun, D., et al. (2018). Cardiac-specific delivery by cardiac tissue-targeting peptide-expressing exosomes. Biochemical and Biophysical Research Communications, 499, 803–808. https://doi.org/10.1016/j.bbrc.2018.03.227
Mentkowski, K. I., & Lang, J. K. (2019). Exosomes engineered to express a cardiomyocyte binding peptide demonstrate improved cardiac retention in vivo. Science and Reports, 9, 10041. https://doi.org/10.1038/s41598-019-46407-1
Chen, C. W., Wang, L. L., Zaman, S., et al. (2018). Sustained release of endothelial progenitor cell-derived extracellular vesicles from shear-thinning hydrogels improves angiogenesis and promotes function after myocardial infarction. Cardiovascular Research, 114, 1029–1040. https://doi.org/10.1093/cvr/cvy067
Acknowledgements
The authors thank Fangfei Wei (from University of Leuven, Belgium) for reviewing and modification of the manuscript.
Funding
The present study was funded by the National Natural Science Foundation of China (Nos. 81770394, 82000260) and Guangdong Basic and Applied Basic Research Foundation (2021A1515010755).
Author information
Authors and Affiliations
Contributions
All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Associate Editor Junjie Xiao oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, H., Xue, R., Huang, P. et al. Modified Exosomes: a Good Transporter for miRNAs within Stem Cells to Treat Ischemic Heart Disease. J. of Cardiovasc. Trans. Res. 15, 514–523 (2022). https://doi.org/10.1007/s12265-022-10216-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-022-10216-1