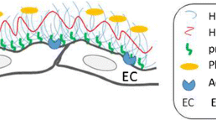
Abstract
Wall shear stress (WSS) plays a key role in maintaining glycocalyx function, gene expression, and structure. Experimental studies have discussed the relationship between the shedding of the endothelial glycocalyx (EG) and WSS. However, rare literature about how WSS affects the EG during cardiopulmonary bypass (CPB) was mentioned. This study aimed to investigate the correlation between the WSS of carotid arteries and shedding of the EG during CPB in humans. The WSS level was calculated in accordance with an equation. The plasma concentrations of heparan sulfate, syndecan-1, and nitric oxide were measured to reflect shedding of the EG at six time points. A negative correlation was observed between the peak wall shear stress (PWSS) and syndecan-1 (R = − 0.5, p < 0.01) and heparan sulfate (R = − 0.461, p < 0.01) during CPB. The WSS is closely associated with the components of glycocalyx shedding during CPB. The WSS produced by non-pulsatile flow during CPB may contribute to the degradation of EG.






Similar content being viewed by others
Change history
12 July 2021
A Correction to this paper has been published: https://doi.org/10.1007/s12265-021-10145-5
Abbreviations
- WSS:
-
Wall shear stress
- PWSS:
-
Peak wall shear stress
- MWSS:
-
Mean wall shear stress
- EG:
-
Endothelial glycocalyx
- CPB:
-
Cardiopulmonary bypass
- HS:
-
Heparan sulfate
- NO:
-
Nitric oxide
- ECG:
-
Electrocardiogram
- ICU:
-
Intensive care unit
- SIRS:
-
Systemic inflammatory reaction syndrome
References
Gao, L., & Lipowsky, H. H. (2010). Composition of the endothelial glycocalyx and its relation to its thickness and diffusion of small solutes. Microvascular Research, 80(3), 394–401. https://doi.org/10.1016/j.mvr.2010.06.005.
Curry, F. E., & Adamson, R. H. (2012). Endothelial glycocalyx: Permeability barrier and mechanosensor. Annals of Biomedical Engineering, 40(4), 828–839. https://doi.org/10.1007/s10439-011-0429-8.
Lipowsky, H. H. (2011). Protease activity and the role of the endothelial glycocalyx in inflammation. Drug Discov Today Dis Models, 8(1), 57–62. https://doi.org/10.1016/j.ddmod.2011.05.004.
Lipowsky, H. H. (2012). The endothelial glycocalyx as a barrier to leukocyte adhesion and its mediation by extracellular proteases. Annals of Biomedical Engineering, 40(4), 840–848. https://doi.org/10.1007/s10439-011-0427-x.
Zhang, J. X., Qu, X. L., Chu, P., Xie, D. J., Zhu, L. L., Chao, Y. L., et al. (2018). Low shear stress induces vascular eNOS uncoupling via autophagy-mediated eNOS phosphorylation. Biochimica et Biophysica Acta, 1865(5), 709–720. https://doi.org/10.1016/j.bbamcr.2018.02.005.
Bartosch, A. M. W., Mathews, R., & Tarbell, J. M. (2017). Endothelial glycocalyx-mediated nitric oxide production in response to selective AFM pulling. Biophysical Journal, 113(1), 101–108. https://doi.org/10.1016/j.bpj.2017.05.033.
Russell-Puleri, S., Dela Paz, N. G., Adams, D., Chattopadhyay, M., Cancel, L., Ebong, E., et al. (2017). Fluid shear stress induces upregulation of COX-2 and PGI2 release in endothelial cells via a pathway involving PECAM-1, PI3K, FAK, and p38. American Journal of Physiology. Heart and Circulatory Physiology, 312(3), H485–H500. https://doi.org/10.1152/ajpheart.00035.2016.
Gouverneur, M., Spaan, J. A., Pannekoek, H., Fontijn, R. D., & Vink, H. (2006). Fluid shear stress stimulates incorporation of hyaluronan into endothelial cell glycocalyx. American Journal of Physiology. Heart and Circulatory Physiology, 290(1), H458–H452. https://doi.org/10.1152/ajpheart.00592.2005.
Nikmanesh, M., Shi, Z. D., & Tarbell, J. M. (2012). Heparan sulfate proteoglycan mediates shear stress-induced endothelial gene expression in mouse embryonic stem cell-derived endothelial cells. Biotechnology and Bioengineering, 109(2), 583–594. https://doi.org/10.1002/bit.23302.
Yang, H., Zhu, L., Chao, Y., Gu, Y., Kong, X., Chen, M., et al. (2018). Hyaluronidase2 (Hyal2) modulates low shear stress-induced glycocalyx impairment via the LKB1/AMPK/NADPH oxidase-dependent pathway. Journal of Cellular Physiology, 233(12), 9701–9715. https://doi.org/10.1002/jcp.26944.
Lipowsky, H. H. (2005). Microvascular rheology and hemodynamics. Microcirculation, 12(1), 5–15. https://doi.org/10.1080/10739680590894966.
Bai, K., & Wang, W. (2014). Shear stress-induced redistribution of the glycocalyx on endothelial cells in vitro. Biomechanics and Modeling in Mechanobiology, 13(2), 303–311. https://doi.org/10.1007/s10237-013-0502-3.
Kong, X., Chen, L., Ye, P., Wang, Z., Zhang, J., Ye, F., et al. (2016). The role of HYAL2 in LSS-induced glycocalyx impairment and the PKA-mediated decrease in eNOS-Ser-633 phosphorylation and nitric oxide production. Molecular Biology of the Cell, 27(25), 3972–3979. https://doi.org/10.1091/mbc.E16-04-0241.
Yang, H., Zhu, L., Gu, Y., Kong, X., Yan, L., Chen, M., et al. (2019). Berberine inhibits low shear stress-induced glycocalyx degradation via modulating AMPK and p47(phox)/Hyal2 signal pathway. European Journal of Pharmacology, 856, 172413. https://doi.org/10.1016/j.ejphar.2019.172413.
Rehm, M., Bruegger, D., Christ, F., Conzen, P., Thiel, M., Jacob, M., et al. (2007). Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation, 116(17), 1896–1906. https://doi.org/10.1161/CIRCULATIONAHA.106.684852.
Kaushal, S., & Wehman, B. (2015). Cardiopulmonary bypass and the endothelial glycocalyx: Shedding new light. The Journal of Thoracic and Cardiovascular Surgery, 150(6), 1482–1483. https://doi.org/10.1016/j.jtcvs.2015.09.071.
Dekker, N. A. M., Veerhoek, D., Koning, N. J., van Leeuwen, A. L. I., Elbers, P. W. G., van den Brom, C. E., et al. (2019). Postoperative microcirculatory perfusion and endothelial glycocalyx shedding following cardiac surgery with cardiopulmonary bypass. Anaesthesia. https://doi.org/10.1111/anae.14577.
Assmann, A., Benim, A. C., Gul, F., Lux, P., Akhyari, P., Boeken, U., et al. (2012). Pulsatile extracorporeal circulation during on-pump cardiac surgery enhances aortic wall shear stress. Journal of Biomechanics, 45(1), 156–163. https://doi.org/10.1016/j.jbiomech.2011.09.021.
Leguy, C. A., Bosboom, E. M., Hoeks, A. P., & van de Vosse, F. N. (2009). Model-based assessment of dynamic arterial blood volume flow from ultrasound measurements. Medical & Biological Engineering & Computing, 47(6), 641–648. https://doi.org/10.1007/s11517-009-0473-9.
Ariff, B. B., Zambanini, A., Sever, P. S., Thom, S. A., & Hughes, A. D. J. A. J. o. H. (2002). The effects of pulsatile flow on shear stress within the carotid artery., 15(4), A68–A68.
Reneman, R. S., van Merode, T., Hick, P., & Hoeks, A. P. (1985). Flow velocity patterns in and distensibility of the carotid artery bulb in subjects of various ages. Circulation, 71(3), 500–509. https://doi.org/10.1161/01.cir.71.3.500.
Lerman, A., & Zeiher, A. M. (2005). Endothelial function: Cardiac events. Circulation, 111(3), 363–368. https://doi.org/10.1161/01.CIR.0000153339.27064.14.
Rubio-Gayosso, I., Platts, S. H., & Duling, B. R. (2006). Reactive oxygen species mediate modification of glycocalyx during ischemia-reperfusion injury. American Journal of Physiology. Heart and Circulatory Physiology, 290(6), H2247–H2256. https://doi.org/10.1152/ajpheart.00796.2005.
Uchimido, R., Schmidt, E. P., & Shapiro, N. I. (2019). The glycocalyx: A novel diagnostic and therapeutic target in sepsis. Critical Care, 23(1), 16. https://doi.org/10.1186/s13054-018-2292-6.
Sangalli, F., Guazzi, M., Senni, S., Sala, W., Caruso, R., Costa, M. C., et al. (2015). Assessing endothelial responsiveness after cardiopulmonary bypass: Insights on different perfusion modalities. Journal of Cardiothoracic and Vascular Anesthesia, 29(4), 912–916. https://doi.org/10.1053/j.jvca.2014.11.008.
Elhadj, S., Mousa, S. A., Biochemistry, K. F.-W. J., & J. o. C. Chronic pulsatile shear stress impacts synthesis of proteoglycans by endothelial cells: Effect on platelet aggregation and coagulation., 86(2), 239–250.
Frati-Munari, A. C. (2013). Medical significance of endothelial glycocalyx. Archivos de Cardiología de México, 83(4), 303–312. https://doi.org/10.1016/j.acmx.2013.04.015.
Becker, B. F., Jacob, M., Leipert, S., Salmon, A. H., & Chappell, D. (2015). Degradation of the endothelial glycocalyx in clinical settings: Searching for the sheddases. British Journal of Clinical Pharmacology, 80(3), 389–402. https://doi.org/10.1111/bcp.12629.
Noble, L. J., Mautes, A. E., & Hall, J. J. (1996). Characterization of the microvascular glycocalyx in normal and injured spinal cord in the rat. The Journal of Comparative Neurology, 376(4), 542–556. https://doi.org/10.1002/(SICI)1096-9861(19961223)376:4<542::AID-CNE4>3.0.CO;2-1.
Potter, D. R., Jiang, J., & Damiano, E. R. (2009). The recovery time course of the endothelial cell glycocalyx in vivo and its implications in vitro. Circulation Research, 104(11), 1318–1325. https://doi.org/10.1161/CIRCRESAHA.108.191585.
Ramnath, R., Foster, R. R., Qiu, Y., Cope, G., Butler, M. J., Salmon, A. H., et al. (2014). Matrix metalloproteinase 9-mediated shedding of syndecan 4 in response to tumor necrosis factor alpha: A contributor to endothelial cell glycocalyx dysfunction. The FASEB Journal, 28(11), 4686–4699. https://doi.org/10.1096/fj.14-252221.
Mulivor, A. W., & Lipowsky, H. H. (2009). Inhibition of glycan shedding and leukocyte-endothelial adhesion in postcapillary venules by suppression of matrixmetalloprotease activity with doxycycline. Microcirculation, 16(8), 657–666. https://doi.org/10.3109/10739680903133714.
Reitsma, S., Slaaf, D. W., Vink, H., van Zandvoort, M. A., & Oude Egbrink, M. G. (2007). The endothelial glycocalyx: Composition, functions, and visualization. Pflügers Archiv, 454(3), 345–359. https://doi.org/10.1007/s00424-007-0212-8.
Chou, P. H., Wang, S. T., Yen, M. H., Liu, C. L., Chang, M. C., & Lee, O. K. (2016). Fluid-induced, shear stress-regulated extracellular matrix and matrix metalloproteinase genes expression on human annulus fibrosus cells. Stem Cell Research & Therapy, 7, 34. https://doi.org/10.1186/s13287-016-0292-5.
Florian, J. A., Kosky, J. R., Ainslie, K., Pang, Z., Dull, R. O., & Tarbell, J. M. (2003). Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circulation Research, 93(10), e136–e142. https://doi.org/10.1161/01.RES.0000101744.47866.D5.
Myers, G. J., & Wegner, J. (2017). Endothelial glycocalyx and cardiopulmonary bypass. The Journal of Extra-Corporeal Technology, 49(3), 174–181.
Sallisalmi, M., Tenhunen, J., Yang, R., Oksala, N., & Pettila, V. (2012). Vascular adhesion protein-1 and syndecan-1 in septic shock. Acta Anaesthesiologica Scandinavica, 56(3), 316–322. https://doi.org/10.1111/j.1399-6576.2011.02578.x.
Kim, Y. H., Nijst, P., Kiefer, K., & Tang, W. H. (2017). Endothelial glycocalyx as biomarker for cardiovascular diseases: Mechanistic and clinical implications. Current Heart Failure Reports, 14(2), 117–126. https://doi.org/10.1007/s11897-017-0320-5.
Flammer, A. J., Anderson, T., Celermajer, D. S., Creager, M. A., Deanfield, J., Ganz, P., et al. (2012). The assessment of endothelial function: From research into clinical practice. Circulation, 126(6), 753–767. https://doi.org/10.1161/CIRCULATIONAHA.112.093245.
Acknowledgment
We wish to thank the Medical Research Collaborating Center, The First Affiliated Hospital of Wenzhou Medical University, for providing statistical consultation services.
Funding
This study was supported by the Wenzhou Science and Technology Bureau of China (No.y20160134).
Author information
Authors and Affiliations
Contributions
(I) Conception and design: GL He and WJ Wang
(II) Administrative support: WJ Wang
(III) Provision of study materials or patients: GL He, LN Lin, and WJ Wang;
(IV) Collection and assembly of data: GL He and LN Lin
(V) Data analysis and interpretation: GL He and WJ Wang
(VI) Manuscript writing: All authors
(VII) Final approval of manuscript: All authors
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
The study protocol was reviewed by our institutional review board and approved as a prospective study (approval number: 2016056). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Associate Editor Junjie Xiao oversaw the review of this article
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: This article was updated after its original publication to add Lina Lin as co-corresponding author (at: wzlinlina@163.com).
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
He, G., Gao, Y., Feng, L. et al. Correlation Between Wall Shear Stress and Acute Degradation of the Endothelial Glycocalyx During Cardiopulmonary Bypass. J. of Cardiovasc. Trans. Res. 13, 1024–1032 (2020). https://doi.org/10.1007/s12265-020-10027-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-020-10027-2