Abstract
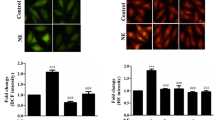
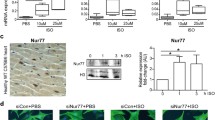
Despite the involvement of ɑ1adrenergic (ɑ1AR) and Histamine 2 receptors (H2R) in cardiac hypertrophy (CH), their relationship is yet to be studied. Our study investigated interrelationship between them using in vitro CH model. H9c2 cardiomyoblasts were exposed to phenylephrine (ɑ1AR agonist-50 μM) in the presence, the absence of famotidine (H2R antagonist-10 μM) and BAY 11-7082 (NF-kB inhibitor-10 μM). The impact of ɑ1AR stimulation on H2R expression and oxidative stress was assessed. Hypertrophic indices were assessed from activities of enzymatic mediators of cardiac hypertrophy, total protein content, BNP levels and cell volume. Additionally, the inverse agonistic property of famotidine and NFkB activity was also studied. ɑ1AR-induced H2R expression, oxidative stress and hypertrophic indices were significantly abolished by famotidine and pharmacological inhibitor of NFkB. Increase in constitutive activity of H2R was noticed correlating with increased receptor population. These results suggest involvement of NFkB-mediated upregulation of H2R in ɑ1AR-mediated CH.







Similar content being viewed by others
Abbreviations
- AR:
-
Adrenergic receptors
- BST:
-
Bryostatin 1
- BUR:
-
Burimamide
- Fam:
-
Famotidine
- H2R:
-
Histamine 2 receptor
- PE:
-
Phenylephrine
References
Triposkiadis, F., Karayannis, G., Giamouzis, G., Skoularigis, J., Louridas, G., & Butler, J. (2009). The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. Journal of the American College of Cardiology, 54(19), 1747–1762. https://doi.org/10.1016/j.jacc.2009.05.015.
Porter, K. E., & Turner, N. A. (2009). Cardiac fibroblasts: At the heart of myocardial remodeling. Pharmacology & Therapeutics, 123(2), 255–278. https://doi.org/10.1016/j.pharmthera.2009.05.002.
Lim, H. W., Windt, L. J. D., Steinberg, L., Taigen, T., Witt, S. A., Kimball, T. R., & Molkentin, J. D. (2000). Calcineurin expression, activation, and function in cardiac pressure-overload hypertrophy. Circulation, 101(20), 2431–2437. https://doi.org/10.1161/01.CIR.101.20.2431.
Cooling, M., Hunter, P., & Crampin, E. J. (2007). Modeling hypertrophic IP3 transients in the cardiac Myocyte. Biophysical Journal, 93(10), 3421–3433. https://doi.org/10.1529/biophysj.107.110031.
Sakata, Y., Hoit, B. D., Liggett, S. B., Walsh, R. A., & Dorn, G. W. (1998). Decompensation of pressure-overload hypertrophy in G alpha q-overexpressing mice. Circulation, 97(15), 1488–1495.
Pönicke, K., Vogelsang, M., Heinroth, M., Becker, K., Zolk, O., Böhm, M., et al. (1998). Endothelin receptors in the failing and nonfailing human heart. Circulation, 97(8), 744–751.
Clark, W. A., Rudnick, S. J., Andersen, L. C., & LaPres, J. J. (1994). Myosin heavy chain synthesis is independently regulated in hypertrophy and atrophy of isolated adult cardiac myocytes. The Journal of Biological Chemistry, 269(41), 25562–25569.
Clark, W. A., Rudnick, S. J., LaPres, J. J., Andersen, L. C., & LaPointe, M. C. (1993). Regulation of hypertrophy and atrophy in cultured adult heart cells. Circulation Research, 73(6), 1163–1176.
Ikeda, U., Tsuruya, Y., & Yaginuma, T. (1991). Alpha 1-adrenergic stimulation is coupled to cardiac myocyte hypertrophy. The American Journal of Physiology, 260(3 Pt 2), H953–H956.
Fuller, S. J., Gaitanaki, C. J., & Sugden, P. H. (1990). Effects of catecholamines on protein synthesis in cardiac myocytes and perfused hearts isolated from adult rats. Stimulation of translation is mediated through the alpha 1-adrenoceptor. The Biochemical Journal, 266(3), 727–736.
Knowlton, K. U., Baracchini, E., Ross, R. S., Harris, A. N., Henderson, S. A., Evans, S. M., et al. (1991). Co-regulation of the atrial natriuretic factor and cardiac myosin light chain-2 genes during alpha-adrenergic stimulation of neonatal rat ventricular cells. Identification of cis sequences within an embryonic and a constitutive contractile protein gene which mediate inducible expression. The Journal of Biological Chemistry, 266(12), 7759–7768.
Bishopric, N. H., Simpson, P. C., & Ordahl, C. P. (1987). Induction of the skeletal alpha-actin gene in alpha 1-adrenoceptor-mediated hypertrophy of rat cardiac myocytes. The Journal of Clinical Investigation, 80(4), 1194–1199. https://doi.org/10.1172/JCI113179.
Kariya, K., Farrance, I. K., & Simpson, P. C. (1993). Transcriptional enhancer factor-1 in cardiac myocytes interacts with an alpha 1-adrenergic- and beta-protein kinase C-inducible element in the rat beta-myosin heavy chain promoter. The Journal of Biological Chemistry, 268(35), 26658–26662.
Long, C. S., Ordahl, C. P., & Simpson, P. C. (1989). Alpha 1-adrenergic receptor stimulation of sarcomeric actin isogene transcription in hypertrophy of cultured rat heart muscle cells. The Journal of Clinical Investigation, 83(3), 1078–1082. https://doi.org/10.1172/JCI113951.
Seddon, M., Looi, Y. H., & Shah, A. M. (2007). Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart, 93(8), 903–907. https://doi.org/10.1136/hrt.2005.068270.
Gaitanaki, C., Konstantina, S., Chrysa, S., & Beis, I. (2003). Oxidative stress stimulates multiple MAPK signalling pathways and phosphorylation of the small HSP27 in the perfused amphibian heart. The Journal of Experimental Biology, 206(Pt 16), 2759–2769.
Heineke, J., & Molkentin, J. D. (2006). Regulation of cardiac hypertrophy by intracellular signalling pathways. Nature reviews. Molecular and Cellular Biology, 7(8), 589–600. https://doi.org/10.1038/nrm1983.
He, H., Liu, X., Lv, L., Liang, H., Leng, B., Zhao, D., et al. (2014). Calcineurin suppresses AMPK-dependent cytoprotective autophagy in cardiomyocytes under oxidative stress. Cell Death & Disease, 5, e997. https://doi.org/10.1038/cddis.2013.533.
Takimoto, E., & Kass, D. A. (2007). Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension, 49(2), 241–248. https://doi.org/10.1161/01.HYP.0000254415.31362.a7.
Xu, Q., Dalic, A., Fang, L., Kiriazis, H., Ritchie, R., Sim, K., et al. (2011). Myocardial oxidative stress contributes to transgenic β2-adrenoceptor activation-induced cardiomyopathy and heart failure. British Journal of Pharmacology, 162(5), 1012–1028. https://doi.org/10.1111/j.1476-5381.2010.01043.x.
Di Lisa, F., Kaludercic, N., & Paolocci, N. (2011). β2–Adrenoceptors, NADPH oxidase, ROS and p38 MAPK: another “radical” road to heart failure? British Journal of Pharmacology, 162(5), 1009–1011. https://doi.org/10.1111/j.1476-5381.2010.01130.x.
Haenen, G. R., Veerman, M., & Bast, A. (1990). Reduction of beta-adrenoceptor function by oxidative stress in the heart. Free Radical Biology & Medicine, 9(4), 279–288.
Hara, M., Ono, K., Hwang, M.-W., Iwasaki, A., Okada, M., Nakatani, K., et al. (2002). Evidence for a role of mast cells in the evolution to congestive heart failure. The Journal of Experimental Medicine, 195(3), 375–381.
Dvorak, A. M. (1986). Mast-cell degranulation in human hearts. The New England Journal of Medicine, 315(15), 969–970.
Luo, T., Chen, B., Zhao, Z., He, N., Zeng, Z., Wu, B., et al. (2013). Histamine H2 receptor activation exacerbates myocardial ischemia/reperfusion injury by disturbing mitochondrial and endothelial function. Basic Research in Cardiology, 108(3), 342. https://doi.org/10.1007/s00395-013-0342-4.
Zeng, Z., Shen, L., Li, X., Luo, T., Wei, X., Zhang, J., et al. (2014). Disruption of histamine H2 receptor slows heart failure progression through reducing myocardial apoptosis and fibrosis. Clinical Science (London, England : 1979), 127(7), 435–448. https://doi.org/10.1042/CS20130716.
Potnuri, A. G., Allakonda, L., Appavoo, A., Saheera, S., & Nair, R. R. (2016). Targeting histamine-2 receptor for prevention of cardiac remodelling in chronic pressure overload. International Journal of Cardiology, 202, 831–833. https://doi.org/10.1016/j.ijcard.2015.10.040.
Takahama, H., Asanuma, H., Sanada, S., Fujita, M., Sasaki, H., Wakeno, M., et al. (2010). A histamine H2 receptor blocker ameliorates development of heart failure in dogs independently of β-adrenergic receptor blockade. Basic Research in Cardiology, 105(6), 787–794. https://doi.org/10.1007/s00395-010-0119-y.
Ahmadi, A., Ebrahimzadeh, M. A., Ahmad-Ashrafi, S., Karami, M., Mahdavi, M. R., & Saravi, S. S. S. (2011). Hepatoprotective, antinociceptive and antioxidant activities of cimetidine, ranitidine and famotidine as histamine H2 receptor antagonists. Fundamental & Clinical Pharmacology, 25(1), 72–79. https://doi.org/10.1111/j.1472-8206.2009.00810.x.
Kesiova, M., Alexandrova, A., Yordanova, N., Kirkova, M., & Todorov, S. (2006). Effects of diphenhydramine and famotidine on lipid peroxidation and activities of antioxidant enzymes in different rat tissues. Pharmacological reports: PR, 58(2), 221–228.
Herman, F. F., Goldberg, B. J., Lad, P. M., Neidzin, H., Kaplan, M., & Easton, J. G. (1990). Effects of histamine on alpha adrenergic receptor expression on the lymphocytes of normal and asthmatic subjects. Annals of Allergy, 65(1), 32–36.
Rius, R. A., Mollner, S., Pfeuffer, T., & Peng Loh, Y. (1994). Developmental changes in Gs and golf proteins and adenylyl cyclases in mouse brain membranes. Brain Research, 643(1–2), 50–58. https://doi.org/10.1016/0006-8993(94)90007-8.
Fraga, C. G., Leibovitz, B. E., & Tappel, A. L. (1988). Lipid peroxidation measured as thiobarbituric acid-reactive substances in tissue slices: characterization and comparison with homogenates and microsomes. Free Radical Biology and Medicine, 4(3), 155–161. https://doi.org/10.1016/0891-5849(88)90023-8.
Weydert, C. J., & Cullen, J. J. (2010). Measurement of superoxide dismutase, catalase, and glutathione peroxidase in cultured cells and tissue. Nature Protocols, 5(1), 51–66. https://doi.org/10.1038/nprot.2009.197.
Li, R., Jen, N., Yu, F., & Hsiai, T. K. (2011). Assessing mitochondrial redox status by flow cytometric methods: vascular response to fluid shear stress. Current protocols in cytometry/editorial board, J. Paul Robinson, managing editor ... [et al.], CHAPTER, Unit9.37. doi:https://doi.org/10.1002/0471142956.cy0937s58.
Eruslanov, E., & Kusmartsev, S. (2010). Identification of ROS using oxidized DCFDA and flow-cytometry. Methods in Molecular Biology (Clifton, N.J.), 594, 57–72. https://doi.org/10.1007/978-1-60761-411-1_4.
Knowles, J. W., Esposito, G., Mao, L., Hagaman, J. R., Fox, J. E., Smithies, O., et al. (2001). Pressure-independent enhancement of cardiac hypertrophy in natriuretic peptide receptor A–deficient mice. Journal of Clinical Investigation, 107(8), 975–984.
Nadruz, W. (2015). Myocardial remodeling in hypertension. Journal of Human Hypertension, 29(1), 1–6. https://doi.org/10.1038/jhh.2014.36.
Aceros, H., Farah, G., Cobos-Puc, L., Stabile, A., Noiseux, N., & Mukaddam-Daher, S. (2011). Moxonidine improves cardiac structure and performance in SHR through inhibition of cytokines, p38 MAPK and Akt. British Journal of Pharmacology, 164(3), 946–957. https://doi.org/10.1111/j.1476-5381.2011.01355.x.
Potnuri, A. G., Allakonda, L., Appavoo, A., Saheera, S., & Nair, R. R. (2018). Association of histamine with hypertension-induced cardiac remodeling and reduction of hypertrophy with the histamine-2-receptor antagonist famotidine compared with the beta-blocker metoprolol. Hypertension Research: Official Journal of the Japanese Society of Hypertension, 41(12), 1023–1035. https://doi.org/10.1038/s41440-018-0109-2.
Kim, J., Ogai, A., Nakatani, S., Hashimura, K., Kanzaki, H., Komamura, K., et al. (2006). Impact of blockade of histamine H2 receptors on chronic heart failure revealed by retrospective and prospective randomized studies. Journal of the American College of Cardiology, 48(7), 1378–1384. https://doi.org/10.1016/j.jacc.2006.05.069.
Khilnani, G., & Khilnani, A. K. (2011). Inverse agonism and its therapeutic significance. Indian Journal of Pharmacology, 43(5), 492–501. https://doi.org/10.4103/0253-7613.84947.
Monczor, F. (2006). Mechanisms of inverse agonism at histamine H(2) receptors - potential benefits and concerns. Inflammopharmacology, 14(1–2), 89–96. https://doi.org/10.1007/s10787-006-1516-6.
Baker, J. G., Hall, I. P., & Hill, S. J. (2003). Agonist and inverse agonist actions of beta-blockers at the human beta 2-adrenoceptor provide evidence for agonist-directed signaling. Molecular Pharmacology, 64(6), 1357–1369. https://doi.org/10.1124/mol.64.6.1357.
Lai, L., Yan, L., Gao, S., Hu, C.-L., Ge, H., Davidow, A., et al. (2013). Type 5 adenylyl cyclase increases oxidative stress by transcriptional regulation of MnSOD via the SIRT1/FoxO3a pathway. Circulation, CIRCULATIONAHA, 113, 001212. https://doi.org/10.1161/CIRCULATIONAHA.112.001212.
Matsushima, S., Ide, T., Yamato, M., Matsusaka, H., Hattori, F., Ikeuchi, M., et al. (2006). Overexpression of mitochondrial peroxiredoxin-3 prevents left ventricular remodeling and failure after myocardial infarction in mice. Circulation, 113(14), 1779–1786. https://doi.org/10.1161/CIRCULATIONAHA.105.582239.
Acknowledgements
The authors would like to acknowledge and show gratitude for the support extended by the National Institute of Pharmaceutical Education and Research, Hyderabad for permitting us to utilize their research facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Statements on Human and Animal Rights and an Informed Consent
The study did not involve any human or animal subject. Hence, statements on Human and Animal Rights and an Informed Consent do not apply.
Additional information
Associate Editor Nicola Smart oversaw the review of this article
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
Raw Western Blot images. (a-b) the pictorial representation of PRX3 and corresponding Beta actin (c-d)) the pictorial representation of H2R and corresponding Beta actin (PNG 1790 kb)

Rights and permissions
About this article
Cite this article
Potnuri, A.G., Allakonda, L. & Saheera, S. Involvement of Histamine 2 Receptor in Alpha 1 Adrenoceptor Mediated Cardiac Hypertrophy and Oxidative Stress in H9c2 Cardio Myoblasts. J. of Cardiovasc. Trans. Res. 14, 184–194 (2021). https://doi.org/10.1007/s12265-020-09967-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-020-09967-6